DCs at the center of help: Origins and evolution of the three-cell-type hypothesis
- PMID: 35543702
- PMCID: PMC9098650
- DOI: 10.1084/jem.20211519
DCs at the center of help: Origins and evolution of the three-cell-type hypothesis
Abstract
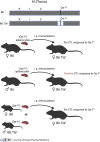
Last year was the 10th anniversary of Ralph Steinman's Nobel Prize awarded for his discovery of dendritic cells (DCs), while next year brings the 50th anniversary of that discovery. Current models of anti-viral and anti-tumor immunity rest solidly on Steinman's discovery of DCs, but also rely on two seemingly unrelated phenomena, also reported in the mid-1970s: the discoveries of "help" for cytolytic T cell responses by Cantor and Boyse in 1974 and "cross-priming" by Bevan in 1976. Decades of subsequent work, controversy, and conceptual changes have gradually merged these three discoveries into current models of cell-mediated immunity against viruses and tumors.
© 2022 Wu and Murphy.
Conflict of interest statement
Disclosures: K.M. Murphy is on the Scientific Advisory Board of Harbour Biomed. No other disclosures were reported.
Figures


References
-
- Ahrends, T., Spanjaard A., Pilzecker B., Babala N., Bovens A., Xiao Y., Jacobs H., and Borst J.. 2017. CD4(+) T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity. 47:848–861.e5. 10.1016/j.immuni.2017.10.009 - DOI - PubMed
-
- Alloatti, A., Kotsias F., Pauwels A.M., Carpier J.M., Jouve M., Timmerman E., Pace L., Vargas P., Maurin M., Gehrmann U., et al. 2015. Toll-like receptor 4 engagement on dendritic cells restrains phago-lysosome fusion and promotes cross-presentation of antigens. Immunity. 43:1087–1100. 10.1016/j.immuni.2015.11.006 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

