Role of adipose tissue macrophages in obesity-related disorders
- PMID: 35543703
- PMCID: PMC9098652
- DOI: 10.1084/jem.20211948
Role of adipose tissue macrophages in obesity-related disorders
Abstract
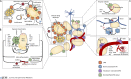
The obesity epidemic has led researchers and clinicians to reconsider the etiology of this disease and precisely decipher its molecular mechanisms. The excessive accumulation of fat by cells, most notably adipocytes, which play a key role in this process, has many repercussions in tissue physiology. Herein, we focus on how macrophages, immune cells well known for their tissue gatekeeping functions, assume fundamental, yet ill-defined, roles in the genesis and development of obesity-related metabolic disorders. We first discuss the determinants of the biology of these cells before introducing the specifics of the adipose tissue environment, while highlighting its heterogeneity. Finally, we detail how obesity transforms both adipose tissue and local macrophage populations. Understanding macrophage diversity and their cross talk with the diverse cell types constituting the adipose tissue environment will allow us to frame the therapeutic potential of adipose tissue macrophages in obesity.
© 2022 Chakarov et al.
Conflict of interest statement
Disclosures: The authors declare no competing financial interests.
Figures


References
-
- Altintas, M.M., Rossetti M.A., Nayer B., Puig A., Zagallo P., Ortega L.M., Johnson K.B., McNamara G., Reiser J., Mendez A.J., and Nayer A.. 2011. Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice. Lipids Health Dis. 10:198. 10.1186/1476-511X-10-198 - DOI - PMC - PubMed
-
- Atabai, K., Jame S., Azhar N., Kuo A., Lam M., McKleroy W., Dehart G., Rahman S., Xia D.D., Melton A.C., et al. . 2009. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J. Clin. Invest. 119:3713–3722. 10.1172/JCI40053 - DOI - PMC - PubMed

