The Function and Structure of the Microsporidia Polar Tube
- PMID: 35544004
- PMCID: PMC10037675
- DOI: 10.1007/978-3-030-93306-7_8
The Function and Structure of the Microsporidia Polar Tube
Abstract
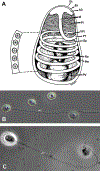
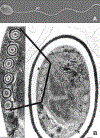
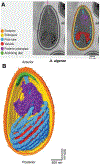
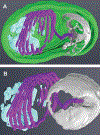
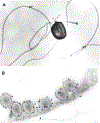
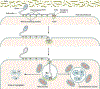
Microsporidia are obligate intracellular pathogens that were initially identified about 160 years ago. Current phylogenetic analysis suggests that they are grouped with Cryptomycota as a basal branch or sister group to the fungi. Microsporidia are found worldwide and can infect a wide range of animals from invertebrates to vertebrates, including humans. They are responsible for a variety of diseases once thought to be restricted to immunocompromised patients but also occur in immunocompetent individuals. The small oval spore containing a coiled polar filament, which is part of the extrusion and invasion apparatus that transfers the infective sporoplasm to a new host, is a defining characteristic of all microsporidia. When the spore becomes activated, the polar filament uncoils and undergoes a rapid transition into a hollow tube that will transport the sporoplasm into a new cell. The polar tube has the ability to increase its diameter from approximately 100 nm to over 600 nm to accommodate the passage of an intact sporoplasm and penetrate the plasmalemma of the new host cell. During this process, various polar tube proteins appear to be involved in polar tube attachment to host cell and can interact with host proteins. These various interactions act to promote host cell infection.
Keywords: Cell-host interaction; Diagnosis; Microsporidia; Microsporidiosis; Polar filament; Polar tube proteins; Spore; Spore wall proteins.
© 2022. The Author(s), under exclusive license to Springer Nature Switzerland AG.
Conflict of interest statement
Conflict of Interest The authors declare that there is no conflict of interest.
Figures






References
-
- Aurrecoechea C, Barreto A, Brestelli J, Brunk BP, Caler EV, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Iodice J, Kissinger JC, Kraemer ET, Li W, Nayak V, Pennington C, Pinney DF, Pitts B, Roos DS, Srinivasamoorthy G, Stoeckert CJ Jr, Treatman C, Wang H (2011) AmoebaDB and MicrosporidiaDB: functional genomic resources for Amoebozoa and microsporidia species. Nucleic Acids Res 39(Database Issue):D612–D619. 10.1093/nar/gkq1006 - DOI - PMC - PubMed
-
- Beznoussenko GV, Dolgikh VV, Seliverstova EV, Semenov PB, Tokarev YS, Trucco A, Micaroni M, Di Giandomenico D, Auinger P, Senderskiy IV, Skarlato SO, Snigirevskaya ES, Komissarchik YY, Pavelka M, De Matteis MA, Luini A, Sokolova YY, Mironov AA (2007) Analogs of the Golgi complex in microsporidia: structure and avesicular mechanisms of function. J Cell Sci 120(Pt 7):1288–1298. 10.1242/jcs.03402 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
