Effect of 3BNC117 and romidepsin on the HIV-1 reservoir in people taking suppressive antiretroviral therapy (ROADMAP): a randomised, open-label, phase 2A trial
- PMID: 35544074
- PMCID: PMC10293654
- DOI: 10.1016/S2666-5247(21)00239-1
Effect of 3BNC117 and romidepsin on the HIV-1 reservoir in people taking suppressive antiretroviral therapy (ROADMAP): a randomised, open-label, phase 2A trial
Abstract
Background: The administration of broadly neutralising anti-HIV-1 antibodies before latency reversal could facilitate elimination of HIV-1-infected CD4 T cells. We tested this concept by combining the broadly neutralising antibody 3BNC117 in combination with the latency-reversing agent romidepsin in people with HIV-1 who were taking suppressive antiretroviral therapy (ART).
Methods: We did a randomised, open-label, phase 2A trial at three university hospital centres in Denmark, Germany, and the USA. Eligible participants were virologically suppressed adults aged 18-65 years who were infected with HIV-1 and on ART for at least 18 months, with plasma HIV-1 RNA concentrations of less than 50 copies per mL for at least 12 months, and a CD4 T-cell count of greater than 500 cells per μL. Participants were randomly assigned (1:1) to receive 3BNC117 plus romidepsin or romidepsin alone in two cycles. All participants received intravenous infusions of romidepsin (5 mg/m2 given over 120 min) at weeks 0, 1, and 2 (treatment cycle 1) and weeks 8, 9, and 10 (treatment cycle 2). Those in the 3BNC117 plus romidepsin group received an intravenous infusion of 3BNC117 (30 mg/kg given over 60 min) 2 days before each treatment cycle. An analytic treatment interruption (ATI) of ART was done at week 24 in both groups. Our primary endpoint was time to viral rebound during analytic treatment interruption, which was assessed in all participants who completed both treatment cycles and ATI. We used a log-rank test to compare time to viral rebound during analytic treatment interruption between the two groups. This trial is registered with ClinicalTrials.gov, NCT02850016. It is closed to new participants, and all follow-up is complete.
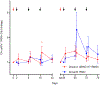
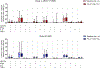
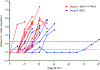
Findings: Between March 20, 2017, and Aug 14, 2018, 22 people were enrolled and randomly assigned, 11 to the 3BNC117 plus romidepsin group and 11 to the romidepsin group. 19 participants completed both treatment cycles and the ATI: 11 in the 3BNC117 plus romidepsin group and 8 in the romidepsin group. The median time to viral rebound during ATI was 18 days (IQR 14-28) in the 3BNC117 plus romidepsin group and 28 days (21-35) in the romidepsin group B (p=0·0016). Although this difference was significant, prolongation of time to viral rebound was not clinically meaningful in either group. All participants in both groups reported adverse events, but overall the combination of 3BNC117 and romidepsin was safe. Two severe adverse events were observed in the romidepsin group during 48 weeks of follow-up, one of which-increased direct bilirubin-was judged to be related to treatment.
Interpretation: The combination of 3BNC117 and romidepsin was safe but did not delay viral rebound during analytic treatment interruptions in individuals on long-term ART. The results of our trial could serve as a benchmark for further optimisation of HIV-1 curative strategies among people with HIV-1 who are taking suppressive ART.
Funding: amfAR, German Center for Infection Research.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests HG and FK are listed as inventors on a patent application for HIV-1 neutralising antibodies filed by the University of Cologne. MCN is listed as an inventor on patents for the antibody 3BNC117. All other authors declare no competing interests.
Figures







References
-
- Rasmussen TA, Tolstrup M, Brinkmann CR, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. lancet HIV. 2014;1(1):e13–21. doi: 10.1016/S2352-3018(14)70014-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

