Single low-dose tafenoquine combined with dihydroartemisinin-piperaquine to reduce Plasmodium falciparum transmission in Ouelessebougou, Mali: a phase 2, single-blind, randomised clinical trial
- PMID: 35544095
- PMCID: PMC9042793
- DOI: 10.1016/S2666-5247(21)00356-6
Single low-dose tafenoquine combined with dihydroartemisinin-piperaquine to reduce Plasmodium falciparum transmission in Ouelessebougou, Mali: a phase 2, single-blind, randomised clinical trial
Erratum in
-
Correction to Lancet Microbe 2022; 3: e336-47.Lancet Microbe. 2022 Oct;3(10):e732. doi: 10.1016/S2666-5247(22)00228-2. Epub 2022 Aug 11. Lancet Microbe. 2022. PMID: 35964635 Free PMC article. No abstract available.
Abstract
Background: Tafenoquine was recently approved as a prophylaxis and radical cure for Plasmodium vivax infection, but its Plasmodium falciparum transmission-blocking efficacy is unclear. We aimed to establish the efficacy and safety of three single low doses of tafenoquine in combination with dihydroartemisinin-piperaquine for reducing gametocyte density and transmission to mosquitoes.
Methods: In this four-arm, single-blind, phase 2, randomised controlled trial, participants were recruited at the Clinical Research Unit of the Malaria Research and Training Centre of the University of Bamako in Mali. Eligible participants were aged 12-50 years, with asymptomatic P falciparum microscopy-detected gametocyte carriage, had a bodyweight of 80 kg or less, and had no clinical signs of malaria defined by fever. Participants were randomly assigned (1:1:1:1) to standard treatment with dihydroartemisinin-piperaquine, or dihydroartemisinin-piperaquine plus a single dose of tafenoquine (in solution) at a final dosage of 0·42 mg/kg, 0·83 mg/kg, or 1·66 mg/kg. Randomisation was done with a computer-generated randomisation list and concealed with sealed, opaque envelopes. Dihydroartemisinin-piperaquine was administered as oral tablets over 3 days (day 0, 1, and 2), as per manufacturer instructions. A single dose of tafenoquine was administered as oral solution on day 0 in parallel with the first dose of dihydroartemisinin-piperaquine. Tafenoquine dosing was based on bodyweight to standardise efficacy and risk variance. The primary endpoint, assessed in the per-protocol population, was median percentage change in mosquito infection rate 7 days after treatment compared with baseline. Safety endpoints included frequency and incidence of adverse events. The final follow-up visit was on Dec 23, 2021; the trial is registered with ClinicalTrials.gov, NCT04609098.
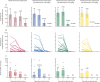
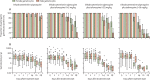
Findings: From Oct 29 to Nov 25, 2020, 1091 individuals were screened for eligibility, 80 of whom were enrolled and randomly assigned (20 per treatment group). Before treatment, 53 (66%) individuals were infectious to mosquitoes, infecting median 12·50% of mosquitoes (IQR 3·64-35·00). Within-group reduction in mosquito infection rate on day 7 was 79·95% (IQR 57·15-100; p=0·0005 for difference from baseline) following dihydroartemisinin-piperaquine only, 100% (98·36-100; p=0·0005) following dihydroartemisinin-piperaquine plus tafenoquine 0·42 mg/kg, 100% (100-100; p=0·0001) following dihydroartemisinin-piperaquine plus tafenoquine 0·83 mg/kg, and 100% (100-100; p=0·0001) following dihydroartemisinin-piperaquine plus tafenoquine 1·66 mg/kg. 55 (69%) of 80 participants had a total of 94 adverse events over the course of the trial; 86 (92%) adverse events were categorised as mild, seven (7%) as moderate, and one (1%) as severe. The most common treatment-related adverse event was mild or moderate headache, which occurred in 15 (19%) participants (dihydroartemisinin-piperaquine n=2; dihydroartemisinin-piperaquine plus tafenoquine 0·42 mg/kg n=6; dihydroartemisinin-piperaquine plus tafenoquine 0·83 mg/kg n=3; and dihydroartemisinin-piperaquine plus tafenoquine 1·66 mg/kg n=4). No serious adverse events occurred. No significant differences in the incidence of all adverse events (p=0·73) or treatment-related adverse events (p=0·62) were observed between treatment groups.
Interpretation: Tafenoquine was well tolerated at all doses and accelerated P falciparum gametocyte clearance. All tafenoquine doses showed improved transmission reduction at day 7 compared with dihydroartemisinin-piperaquine alone. These data support the case for further research on tafenoquine as a transmission-blocking supplement to standard antimalarials.
Funding: Bill & Melinda Gates Foundation.
Translations: For the French, Portuguese, Spanish and Swahili translations of the abstract see Supplementary Materials section.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Is low-dose tafenoquine combined with dihydroartemisinin-piperaquine a potential risk factor for Plasmodium vivax resistance to 8-aminoquinolines?Lancet Microbe. 2022 Jul;3(7):e477. doi: 10.1016/S2666-5247(22)00113-6. Epub 2022 Apr 26. Lancet Microbe. 2022. PMID: 35779563 No abstract available.
References
-
- WHO . World Health Organization; Geneva, Switzerland: 2020. World Malaria Report 2020.
-
- WHO . World Health Organization; Geneva, Switzerland: 2015. Global Malaria Programme: WHO policy brief on single-dose primaquine as a gametocytocide in Plasmodium falciparum malaria.
-
- Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–1345. - PubMed
-
- Frampton JE. Tafenoquine: first global approval. Drugs. 2018;78:1517–1523. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

