Comparison of Hepatic Arterial Infusion Pump Chemotherapy vs Resection for Patients With Multifocal Intrahepatic Cholangiocarcinoma
- PMID: 35544131
- PMCID: PMC9096688
- DOI: 10.1001/jamasurg.2022.1298
Comparison of Hepatic Arterial Infusion Pump Chemotherapy vs Resection for Patients With Multifocal Intrahepatic Cholangiocarcinoma
Abstract
Importance: Intrahepatic cholangiocarcinoma (iCCA) is often multifocal (ie, satellites or intrahepatic metastases) at presentation.
Objective: To compare the overall survival (OS) of patients with multifocal iCCA after hepatic arterial infusion pump (HAIP) floxuridine chemotherapy vs resection.
Design, setting, and participants: In this cohort study, patients with histologically confirmed, multifocal iCCA were eligible. The HAIP group consisted of consecutive patients from a single center who underwent HAIP floxuridine chemotherapy for unresectable multifocal iCCA between January 1, 2001, and December 31, 2018. The resection group consisted of consecutive patients from 12 centers who underwent a curative-intent resection for multifocal iCCA between January 1, 1990, and December 31, 2017. Resectable metastatic disease to regional lymph nodes and previous systemic therapy were permitted. Patients with distant metastatic disease (ie, stage IV), those who underwent resection before starting HAIP floxuridine chemotherapy, and those who received a liver transplant were excluded. Data were analyzed on September 1, 2021.
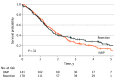
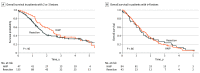
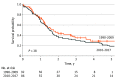
Main outcomes and measures: Overall survival in the 2 treatment groups was compared using the Kaplan-Meier method and log-rank test.
Results: A total of 319 patients with multifocal iCCA were included: 141 in the HAIP group (median [IQR] age, 62 [53-70] years; 79 [56.0%] women) and 178 in the resection group (median [IQR] age, 60 [50-69] years; 91 [51.1%] men). The HAIP group was characterized by a higher percentage of bilobar disease (88.0% [n = 124] vs 34.3% [n = 61]), larger tumors (median, 8.4 cm vs 7.0 cm), and a higher proportion of patients with 4 or more lesions (66.7% [94] vs 24.2% [43]). Postoperative mortality after 30 days was 0.8% (95% CI, 0.0%-2.1%) in the HAIP group vs 6.2% (95% CI, 2.3%-9.7%) in the resection group (P = .01). The median OS for HAIP was 20.3 months vs 18.9 months for resection (P = .32). Five-year OS in patients with 2 or 3 lesions was 23.7% (95% CI, 12.3%-45.7%) in the HAIP group vs 25.7% (95% CI, 17.9%-37.0%) in the resection group. Five-year OS in patients with 4 or more lesions was 5.0% (95% CI, 1.7%-14.3%) in the HAIP group vs 6.8% (95% CI, 1.8%-25.3%) in the resection group. After adjustment for tumor diameter, number of tumors, and lymph node metastases, the hazard ratio of HAIP vs resection was 0.75 (95% CI, 0.55-1.03; P = .07).
Conclusions and relevance: This cohort study found that patients with multifocal iCCA had similar OS after HAIP floxuridine chemotherapy vs resection. Resection of multifocal intrahepatic cholangiocarcinoma needs to be considered carefully given the complication rate of major liver resection; HAIP floxuridine chemotherapy may be an effective alternative option.
Conflict of interest statement
Figures
Comment in
-
Multifocal Intrahepatic Cholangiocarcinoma and Operative Management of Inoperable Disease.JAMA Surg. 2022 Jul 1;157(7):597. doi: 10.1001/jamasurg.2022.1305. JAMA Surg. 2022. PMID: 35544210 No abstract available.
-
Treatment for Patients With Multifocal Intrahepatic Cholangiocarcinoma-Reply.JAMA Surg. 2023 Mar 1;158(3):327. doi: 10.1001/jamasurg.2022.4460. JAMA Surg. 2023. PMID: 36383349 No abstract available.
-
Treatment for Patients With Multifocal Intrahepatic Cholangiocarcinoma.JAMA Surg. 2023 Mar 1;158(3):325-326. doi: 10.1001/jamasurg.2022.4458. JAMA Surg. 2023. PMID: 36383355 No abstract available.
-
Treatment for Patients With Multifocal Intrahepatic Cholangiocarcinoma.JAMA Surg. 2023 Mar 1;158(3):326-327. doi: 10.1001/jamasurg.2022.4459. JAMA Surg. 2023. PMID: 36383372 No abstract available.