Application of Patient-Reported Outcome Measurements in Clinical Trials in China
- PMID: 35544134
- PMCID: PMC9096600
- DOI: 10.1001/jamanetworkopen.2022.11644
Application of Patient-Reported Outcome Measurements in Clinical Trials in China
Abstract
Importance: Regulatory authorities, industry peers, and international health policies have emphasized the value of assessing patient-reported outcomes (PROs) in clinical studies. Despite the increase in the number of clinical studies in the last decade in China, little is known about the extent of the use of PROs.
Objective: To evaluate the application and characteristics of PRO instruments as primary and secondary outcomes in randomized clinical trials in China.
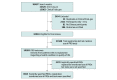
Design, setting, and participants: A cross-sectional study of interventional clinical trials conducted in China from January 1, 2010, to December 31, 2020, was performed. Data obtained from the Chinese Clinical Trial Registry and ClinicalTrials.gov databases were evaluated.
Main outcomes and measures: Trials were categorized according to those that (1) precisely listed PRO tools as outcomes, (2) mentioned patient subjective feelings in outcomes but did not clarify which tools were used for assessment, and (3) did not mention any PRO measurements. Data on study phase, setting, participant age, and sex were extracted from trials that considered patient feelings, along with the target diseases and names of the PRO tools.
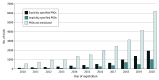
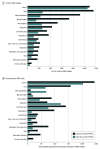
Results: Among a total of 34 033 trials, 6915 (20.3%) listed the explicit PRO instruments used and 3178 (9.3%) included PRO in their outcomes but did not include the names of the assessment tools. From more than 32 million people included in the registered trials, data on 1.5 million (4.7%) patients were scientifically collected by PRO instruments, and subjective feelings were assessed for 693 867 (2.1%) participants. Pain (16.8%), cancer (15.6%), and musculoskeletal symptoms (13.3%) were the most common conditions for which PROs were precisely collected by tools. The most common tools for PRO measurements were the visual analog scale, Short-Form 36, and Hamilton Depression Scale.
Conclusions and relevance: In this cross-sectional study, the use of PROs increased during the study period in clinical trials conducted in China. However, patient opinion appears to still be rarely measured. The application of PRO is geographically unevenly distributed. Development of PRO instruments, especially those suitable for the Chinese population, may be useful. Further expansion of PROs with respect to the scope of diseases is needed to avoid missing important data.
Conflict of interest statement
Figures
Comment in
-
Patient-Reported Outcome Measures-Challenges and Opportunities for China.JAMA Netw Open. 2022 May 2;5(5):e2211652. doi: 10.1001/jamanetworkopen.2022.11652. JAMA Netw Open. 2022. PMID: 35544140 No abstract available.
Similar articles
-
Application of Patient-Reported Outcome Measurements in Adult Tumor Clinical Trials in China: Cross-Sectional Study.J Med Internet Res. 2024 May 8;26:e45719. doi: 10.2196/45719. J Med Internet Res. 2024. PMID: 38718388 Free PMC article.
-
A cross-sectional study on the application of patient-reported outcome measurements in clinical trials of traditional Chinese medicine in mainland China.Front Pharmacol. 2023 May 11;14:1159906. doi: 10.3389/fphar.2023.1159906. eCollection 2023. Front Pharmacol. 2023. PMID: 37251323 Free PMC article.
-
Patient-reported outcomes and target effect sizes in pragmatic randomized trials in ClinicalTrials.gov: A cross-sectional analysis.PLoS Med. 2022 Feb 8;19(2):e1003896. doi: 10.1371/journal.pmed.1003896. eCollection 2022 Feb. PLoS Med. 2022. PMID: 35134080 Free PMC article. Review.
-
Trials with patient-reported outcomes registered on the Australian New Zealand Clinical Trials Registry (ANZCTR).Qual Life Res. 2018 Oct;27(10):2581-2591. doi: 10.1007/s11136-018-1921-5. Epub 2018 Jun 18. Qual Life Res. 2018. PMID: 29915979
-
Rare use of patient-reported outcomes in childhood cancer clinical trials - a systematic review of clinical trial registries.Eur J Cancer. 2021 Jul;152:90-99. doi: 10.1016/j.ejca.2021.04.023. Epub 2021 Jun 2. Eur J Cancer. 2021. PMID: 34090144
Cited by
-
Neurosurgical nurses' perspectives of patient-reported outcomes: a qualitative study.BMC Nurs. 2025 Aug 11;24(1):1047. doi: 10.1186/s12912-025-03726-1. BMC Nurs. 2025. PMID: 40790591 Free PMC article.
-
Translation, Cross-Cultural Adaptation and Validation of the Chinese Version of the High Activity Arthroplasty Score.Patient Relat Outcome Meas. 2024 Apr 29;15:121-130. doi: 10.2147/PROM.S451710. eCollection 2024. Patient Relat Outcome Meas. 2024. PMID: 38706693 Free PMC article.
-
Impact of Internet Addiction on Patient-Reported Outcomes in Chinese Children Aged 8 to 18 Years With Malignant Tumors: A Cross-Sectional Study.Cureus. 2025 Apr 26;17(4):e83038. doi: 10.7759/cureus.83038. eCollection 2025 Apr. Cureus. 2025. PMID: 40290557 Free PMC article.
-
Effects of WeChat-based telehealth on patients reported outcomes of hypertension: a randomized controlled trial.Sci Rep. 2025 Jul 1;15(1):22035. doi: 10.1038/s41598-025-04504-4. Sci Rep. 2025. PMID: 40596064 Free PMC article. Clinical Trial.
-
Differences and common ground in the frameworks of health-related quality of life in traditional Chinese medicine and modern medicine: a systematic review.Qual Life Res. 2024 Jul;33(7):1795-1806. doi: 10.1007/s11136-024-03669-1. Epub 2024 May 13. Qual Life Res. 2024. PMID: 38740639 Free PMC article.
References
-
- Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. US Food and Drug Administration; 2009.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous