Gabapentin as Add-On to Fentanyl and Midazolam in Patients Receiving Mechanical Ventilation: A Randomized, Blinded Study
- PMID: 35544248
- PMCID: PMC9361057
- DOI: 10.5152/TJAR.2022.21366
Gabapentin as Add-On to Fentanyl and Midazolam in Patients Receiving Mechanical Ventilation: A Randomized, Blinded Study
Abstract
Objective: Fentanyl and midazolam are popular drugs for sedation and analgesia in intensive care unit. Gabapentin has sedative and analgesic effects, as well. Our purpose was to study gabapentin addition to fentanyl and midazolam to reach the target sedation level in patients requiring mechanical ventilation.
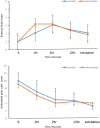
Methods: This was a randomized and double-blinded trial. Fifty patients receiving mechanical ventilation and aged from 18 to 70 years were randomized 1 : 1 to 300 mg gabapentin q8hr or placebo. The initial infusion rates of fentanyl and midazolam were 1-2 µg kg-1 h-1 and 0.06-0.2 mg kg-1 h-1, respectively, in both groups. Treatments continued prior to weaning. Ramsay sedation scale score (2-3) and behavioral pain scale score (≤4) were used for the evaluation of sedation and analgesia levels, respectively.
Results: A total of 43 patients were studied. Both treatment modalities reached the target sedation and analgesia levels. In the intervention group, there were significant reductions in daily consumption of fentanyl and midazolam (P < .01). Duration of ventilation was shorter in the intervention group (4.1 ± 1.7 days vs 5.1 ± 1.3 days, P > .05). There was no significant difference in intensive care hospitalization, although it was shorter in the intervention group (201 ± 24 hours vs 224 ± 19 hours, P > .05).
Conclusions: This trail showed that both treatment modalities could reach target sedation and analgesia levels without significant differences. Add-on therapy with gabapentin could reduce the total dose of fentanyl and midazolam.
Figures
References
LinkOut - more resources
Full Text Sources