Metathesis between E-C(spn ) and H-C(sp3 ) σ-Bonds (E=Si, Ge; n=2, 3) on an Osmium-Polyhydride
- PMID: 35544362
- PMCID: PMC9401005
- DOI: 10.1002/anie.202204081
Metathesis between E-C(spn ) and H-C(sp3 ) σ-Bonds (E=Si, Ge; n=2, 3) on an Osmium-Polyhydride
Abstract
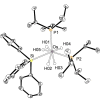
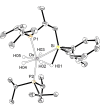
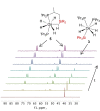
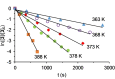
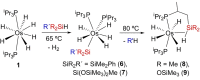
The silylation of a phosphine of OsH6 (Pi Pr3 )2 is performed via net-metathesis between Si-C(spn ) and H-C(sp3 ) σ-bonds (n=2, 3). Complex OsH6 (Pi Pr3 )2 activates the Si-H bond of Et3 SiH and Ph3 SiH to give OsH5 (SiR3 )(Pi Pr3 )2 , which yield OsH4 {κ1 -P,η2 -SiH-[i Pr2 PCH(Me)CH2 SiR2 H]}(Pi Pr3 ) and R-H (R=Et, Ph), by displacement of a silyl substituent with a methyl group of a phosphine. Such displacement is a first-order process, with activation entropy consistent with a rate determining step occurring via a highly ordered transition state. It displays selectivity, releasing the hydrocarbon resulting from the rupture of the weakest Si-substituent bond, when the silyl ligand bears different substituents. Accordingly, reactions of OsH6 (Pi Pr3 )2 with dimethylphenylsilane, and 1,1,1,3,5,5,5-heptamethyltrisiloxane afford OsH5 (SiR2 R')(Pi Pr3 )2 , which evolve into OsH4 {κ1 -P,η2 -GeH-[i Pr2 PCH(Me)CH2 SiR2 H]}(Pi Pr3 ) (R=Me, OSiMe3 ) and R'-H (R'=Ph, Me). Exchange reaction is extended to Et3 GeH. The latter reacts with OsH6 (Pi Pr3 )2 to give OsH5 (GeEt3 )(Pi Pr3 )2 , which loses ethane to form OsH4 {κ1 -P,η2 -GeH-[i Pr2 PCH(Me)CH2 GeEt2 H]}(Pi Pr3 ).
Keywords: Germanes; Osmium; Polyhydrides; Silanes; σ-Bond Metathesis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

