Evaluation of mRNA-1273 Covid-19 Vaccine in Children 6 to 11 Years of Age
- PMID: 35544369
- PMCID: PMC9127699
- DOI: 10.1056/NEJMoa2203315
Evaluation of mRNA-1273 Covid-19 Vaccine in Children 6 to 11 Years of Age
Abstract
Background: Vaccination of children to prevent coronavirus disease 2019 (Covid-19) is an urgent public health need. The safety, immunogenicity, and efficacy of the mRNA-1273 vaccine in children 6 to 11 years of age are unknown.
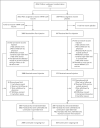
Methods: Part 1 of this ongoing phase 2-3 trial was open label for dose selection; part 2 was an observer-blinded, placebo-controlled expansion evaluation of the selected dose. In part 2, we randomly assigned children (6 to 11 years of age) in a 3:1 ratio to receive two injections of mRNA-1273 (50 μg each) or placebo, administered 28 days apart. The primary objectives were evaluation of the safety of the vaccine in children and the noninferiority of the immune response in these children to that in young adults (18 to 25 years of age) in a related phase 3 trial. Secondary objectives included determination of the incidences of confirmed Covid-19 and severe acute respiratory syndrome coronavirus 2 infection, regardless of symptoms. Interim analysis results are reported.
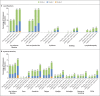
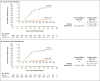
Results: In part 1 of the trial, 751 children received 50-μg or 100-μg injections of the mRNA-1273 vaccine, and on the basis of safety and immunogenicity results, the 50-μg dose level was selected for part 2. In part 2 of the trial, 4016 children were randomly assigned to receive two injections of mRNA-1273 (50 μg each) or placebo and were followed for a median of 82 days (interquartile range, 14 to 94) after the first injection. This dose level was associated with mainly low-grade, transient adverse events, most commonly injection-site pain, headache, and fatigue. No vaccine-related serious adverse events, multisystem inflammatory syndrome in children, myocarditis, or pericarditis were reported as of the data-cutoff date. One month after the second injection (day 57), the neutralizing antibody titer in children who received mRNA-1273 at a 50-μg level was 1610 (95% confidence interval [CI], 1457 to 1780), as compared with 1300 (95% CI, 1171 to 1443) at the 100-μg level in young adults, with serologic responses in at least 99.0% of the participants in both age groups, findings that met the prespecified noninferiority success criterion. Estimated vaccine efficacy was 88.0% (95% CI, 70.0 to 95.8) against Covid-19 occurring 14 days or more after the first injection, at a time when B.1.617.2 (delta) was the dominant circulating variant.
Conclusions: Two 50-μg doses of the mRNA-1273 vaccine were found to be safe and effective in inducing immune responses and preventing Covid-19 in children 6 to 11 years of age; these responses were noninferior to those in young adults. (Funded by the Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases; KidCOVE ClinicalTrials.gov number, NCT04796896.).
Copyright © 2022 Massachusetts Medical Society.
Figures
References
-
- Food and Drug Administration. Coronavirus (COVID-19) update: FDA takes key action by approving second covid-19 vaccine. Press release, January 31, 2022. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...).
-
- Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis 2021;72(9):e206-e214. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
