Variation in Glucose-6-Phosphate Dehydrogenase activity following acute malaria
- PMID: 35544453
- PMCID: PMC9094517
- DOI: 10.1371/journal.pntd.0010406
Variation in Glucose-6-Phosphate Dehydrogenase activity following acute malaria
Abstract
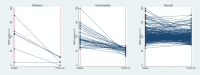
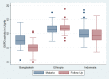
Primaquine and tafenoquine are the only licensed drugs with activity against Plasmodium vivax hypnozoites but cause haemolysis in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. Malaria also causes haemolysis, leading to the replacement of older erythrocytes with low G6PD activity by reticulocytes and young erythrocytes with higher activity. Aim of this study was to assess the impact of acute malaria on G6PD activity. Selected patients with uncomplicated malaria were recruited in Bangladesh (n = 87), Indonesia (n = 75), and Ethiopia (n = 173); G6PD activity was measured at the initial presentation with malaria and a median of 176 days later (range 140 to 998) in the absence of malaria. Among selected participants (deficient participants preferentially enrolled in Bangladesh but not at other sites) G6PD activity fell between malaria and follow up by 79.1% (95%CI: 40.4 to 117.8) in 6 participants classified as deficient (<30% activity), 43.7% (95%CI: 34.2 to 53.1) in 39 individuals with intermediate activity (30% to <70%), and by 4.5% (95%CI: 1.4 to 7.6) in 290 G6PD normal (≥70%) participants. In Bangladesh and Indonesia G6PD activity was significantly higher during acute malaria than when the same individuals were retested during follow up (40.9% (95%CI: 33.4-48.1) and 7.4% (95%CI: 0.2 to 14.6) respectively), whereas in Ethiopia G6PD activity was 3.6% (95%CI: -1.0 to -6.1) lower during acute malaria. The change in G6PD activity was apparent in patients presenting with either P. vivax or P. falciparum infection. Overall, 66.7% (4/6) severely deficient participants and 87.2% (34/39) with intermediate deficiency had normal activities when presenting with malaria. These findings suggest that G6PD activity rises significantly and at clinically relevant levels during acute malaria. Prospective case-control studies are warranted to confirm the degree to which the predicted population attributable risks of drug induced haemolysis is lower than would be predicted from cross sectional surveys.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
G6PD Deficiency and Antimalarial Efficacy for Uncomplicated Malaria in Bangladesh: A Prospective Observational Study.PLoS One. 2016 Apr 29;11(4):e0154015. doi: 10.1371/journal.pone.0154015. eCollection 2016. PLoS One. 2016. PMID: 27128675 Free PMC article.
-
Prospective observational study to assess the feasibility and safety of appropriate Plasmodium vivax radical cure with tafenoquine or primaquine after quantitative G6PD testing during pilot implementation in Thailand.BMJ Glob Health. 2025 Apr 24;10(4):e016720. doi: 10.1136/bmjgh-2024-016720. BMJ Glob Health. 2025. PMID: 40274286 Free PMC article.
-
Haemolysis in G6PD Heterozygous Females Treated with Primaquine for Plasmodium vivax Malaria: A Nested Cohort in a Trial of Radical Curative Regimens.PLoS Med. 2017 Feb 7;14(2):e1002224. doi: 10.1371/journal.pmed.1002224. eCollection 2017 Feb. PLoS Med. 2017. PMID: 28170391 Free PMC article. Clinical Trial.
-
Primaquine alternative dosing schedules for preventing malaria relapse in people with Plasmodium vivax.Cochrane Database Syst Rev. 2020 Aug 19;8:CD012656. doi: 10.1002/14651858.CD012656.pub3. Cochrane Database Syst Rev. 2020. PMID: 32816320
-
Tafenoquine for preventing relapse in people with Plasmodium vivax malaria.Cochrane Database Syst Rev. 2020 Sep 6;9(9):CD010458. doi: 10.1002/14651858.CD010458.pub3. Cochrane Database Syst Rev. 2020. PMID: 32892362 Free PMC article.
Cited by
-
Performance of quantitative point-of-care tests to measure G6PD activity: An individual participant data meta-analysis.PLoS Negl Trop Dis. 2025 Mar 25;19(3):e0012864. doi: 10.1371/journal.pntd.0012864. eCollection 2025 Mar. PLoS Negl Trop Dis. 2025. PMID: 40132008 Free PMC article.
-
Primaquine dose and the risk of haemolysis in patients with uncomplicated Plasmodium vivax malaria: a systematic review and individual patient data meta-analysis.Lancet Infect Dis. 2024 Feb;24(2):184-195. doi: 10.1016/S1473-3099(23)00431-0. Epub 2023 Sep 22. Lancet Infect Dis. 2024. PMID: 37748497 Free PMC article.
-
[Malaria control in French Guiana: What are the challenges in this last endemic French territory in 2024?].Med Trop Sante Int. 2025 Mar 20;5(1):mtsi.v5i1.2025.536. doi: 10.48327/mtsi.v5i1.2025.536. eCollection 2025 Mar 31. Med Trop Sante Int. 2025. PMID: 40248577 Free PMC article. French.
-
Genetic Variants of Glucose-6-Phosphate Dehydrogenase and Their Associated Enzyme Activity: A Systematic Review and Meta-Analysis.Pathogens. 2022 Sep 14;11(9):1045. doi: 10.3390/pathogens11091045. Pathogens. 2022. PMID: 36145477 Free PMC article. Review.
-
A Review of the Current Status of G6PD Deficiency Testing to Guide Radical Cure Treatment for Vivax Malaria.Pathogens. 2023 Apr 27;12(5):650. doi: 10.3390/pathogens12050650. Pathogens. 2023. PMID: 37242320 Free PMC article. Review.
References
-
- Dini S, Douglas NM, Poespoprodjo JR, Kenangalem E, Sugiarto P, Plumb ID, et al.. The risk of morbidity and mortality following recurrent malaria in Papua, Indonesia: a retrospective cohort study. BMC Med. 2020;18(1):28. doi: 10.1186/s12916-020-1497-0 ; PubMed Central PMCID: PMC7031957. - DOI - PMC - PubMed
-
- Thriemer K, Ley B, Bobogare A, Dysoley L, Alam MS, Pasaribu AP, et al.. Challenges for achieving safe and effective radical cure of Plasmodium vivax: a round table discussion of the APMEN Vivax Working Group. Malaria journal. 2017;16(1):141. doi: 10.1186/s12936-017-1784-1 ; PubMed Central PMCID: PMC5382417. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous