Hepatitis C prevalence and key population size estimate updates in San Francisco: 2015 to 2019
- PMID: 35544483
- PMCID: PMC9094540
- DOI: 10.1371/journal.pone.0267902
Hepatitis C prevalence and key population size estimate updates in San Francisco: 2015 to 2019
Abstract
Background: In 2017, San Francisco's initiative to locally eliminate hepatitis C virus (HCV) as a public health threat, End Hep C SF, generated an estimate of city-wide HCV prevalence in 2015, but only incorporated limited information about population HCV treatment. Using additional data and updated methods, we aimed to update the 2015 estimate to 2019 and provide a more accurate estimate of the number of people with untreated, active HCV infection overall and in key subgroups-people who inject drugs (PWID), men who have sex with men (MSM), and low socioeconomic status transgender women (low SES TW).
Methods: Our estimates are based on triangulation of data from blood bank testing records, cross-sectional and longitudinal observational studies, and published literature. We calculated subpopulation estimates based on biological sex, age and/or HCV risk group. When multiple sources of data were available for subpopulation estimates, we calculated an average using inverse variance weighting. Plausible ranges (PRs) were conservatively estimated to convey uncertainty.
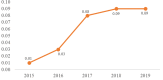
Results: The total number of people estimated to have anti-HCV antibodies in San Francisco in 2019 was 22,585 (PR:12,014-44,152), with a citywide seroprevalence of 2.6% (PR:1.4%-5.0%)-similar to the 2015 estimate of 21,758 (PR:10,274-42,067). Of all people with evidence of past or present infection, an estimated 11,582 (PR:4,864-35,094) still had untreated, active HCV infection, representing 51.3% (PR:40.5%-79.5%) of all people with anti-HCV antibodies, and 1.3% (PR:0.6%-4.0%) of all San Franciscans. PWID comprised an estimated 2.8% of the total population of San Francisco, yet 73.1% of people with anti-HCV antibodies and 90.4% (n = 10,468, PR:4,690-17,628) of untreated, active HCV infections were among PWID. MSM comprised 7.8% of the total population, yet 11.7% of people with anti-HCV antibodies and 1.0% (n = 119, PR:0-423) of those with untreated active infections. Low SES TW comprised an estimated 0.1% of the total population, yet 1.4% of people with HCV antibodies and 1.6% (n = 183, PR:130-252) of people with untreated active infections.
Conclusions: Despite the above-average number (2.6%) of people with anti-HCV antibodies, we estimate that only 1.3% (PR:0.6%-4.0%) of all San Francisco residents have untreated, active HCV infection-likely a reflection of San Francisco's robust efforts to diagnose infection among high-risk groups and initiate curative treatment with as many people as possible. While plausible ranges of infections are wide, these findings indicate that while the overall number of people with anti-HCV antibodies may have increased slightly, the number of people with active HCV infection may have decreased slightly since 2015. This estimate improves upon the 2015 calculations by directly estimating the impact of curative treatment citywide and in subgroups. However, more research is needed to better understand the burden of HCV disease among other subgroups at high risk, such as Blacks/African Americans, people with a history of injection drug use (but not injecting drugs in the last 12 months), people who are currently or formerly incarcerated, and people who are currently or formerly unhoused.
Conflict of interest statement
Shelley N. Facente is affiliated to Facente Consulting. Facente Consulting provided support in the form of salary for author S.N.F., but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Authors Shelley N. Facente and Eduard Grebe have received consulting fees and research support for unrelated work from Gilead Sciences. There are no patents, products in development or marketed products to declare. End Hep C SF has received donations from the Gilead Foundation and the AbbVie Foundation, but those donations are unrelated to the current work and no direct funding was received by any of the co-authors from these donations. None of these interests alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Estimated hepatitis C prevalence and key population sizes in San Francisco: A foundation for elimination.PLoS One. 2018 Apr 11;13(4):e0195575. doi: 10.1371/journal.pone.0195575. eCollection 2018. PLoS One. 2018. PMID: 29641546 Free PMC article.
-
Hepatitis C Care Cascades for 3 Populations at High Risk: Low-income Trans Women, Young People Who Inject Drugs, and Men Who Have Sex With Men and Inject Drugs.Clin Infect Dis. 2021 Sep 15;73(6):e1290-e1295. doi: 10.1093/cid/ciab261. Clin Infect Dis. 2021. PMID: 33768236 Free PMC article.
-
Impact of HCV Testing and Treatment on HCV Transmission Among Men Who Have Sex With Men and Who Inject Drugs in San Francisco: A Modelling Analysis.J Infect Dis. 2023 Sep 15;228(6):662-673. doi: 10.1093/infdis/jiad169. J Infect Dis. 2023. PMID: 37486337 Free PMC article.
-
Seroprevalence and burden of hepatitis C virus infection in WHO South-East Asia Region: A systematic review.J Gastroenterol Hepatol. 2022 Jun;37(6):964-972. doi: 10.1111/jgh.15827. Epub 2022 Mar 18. J Gastroenterol Hepatol. 2022. PMID: 35263807
-
Incidence of HIV and hepatitis C virus among people who inject drugs, and associations with age and sex or gender: a global systematic review and meta-analysis.Lancet Gastroenterol Hepatol. 2023 Jun;8(6):533-552. doi: 10.1016/S2468-1253(23)00018-3. Epub 2023 Mar 27. Lancet Gastroenterol Hepatol. 2023. PMID: 36996853 Free PMC article.
Cited by
-
Modeling the impact of the COVID-19 pandemic on achieving HCV elimination amongst young and unstably housed people who inject drugs in San Francisco.Int J Drug Policy. 2024 Sep;131:104452. doi: 10.1016/j.drugpo.2024.104452. Epub 2024 Jun 22. Int J Drug Policy. 2024. PMID: 38910096 Free PMC article.
-
Getting to full disclosure: HCV testing and status disclosure behaviors among PWID and their injecting partners.BMC Public Health. 2025 May 8;25(1):1707. doi: 10.1186/s12889-025-22781-6. BMC Public Health. 2025. PMID: 40340694 Free PMC article.
-
A single-site randomized controlled trial of partner navigation to HCV treatment for people who inject drugs: a study protocol for the You're Empowered for Treatment Initiation (YETI) partner trial.Trials. 2025 Jan 22;26(1):26. doi: 10.1186/s13063-024-08662-0. Trials. 2025. PMID: 39844334 Free PMC article.
References
-
- World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. Geneva: Switzerland; 2016 May.
-
- Buckley GL, Strom BL, (eds). A National Strategy for the Elimination of Hepatitis B and C. Washington, D.C.: The National Academicies of Sciences, Engineering, and Medicine; 2017. March 28.
-
- New York State Hepatitis C Elimination Campaign. End Hep C NY 2017 [Available from: https://www.endhepcny.org/].
-
- End Hep C SF. End Hep C SF Strategic Plan, 2017–2019. San Francisco, CA; 2017.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous