Real-time 3D optoacoustic tracking of cell-sized magnetic microrobots circulating in the mouse brain vasculature
- PMID: 35544570
- PMCID: PMC9094653
- DOI: 10.1126/sciadv.abm9132
Real-time 3D optoacoustic tracking of cell-sized magnetic microrobots circulating in the mouse brain vasculature
Abstract
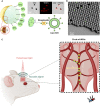
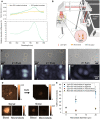
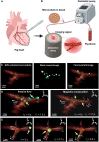
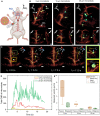
Mobile microrobots hold remarkable potential to revolutionize health care by enabling unprecedented active medical interventions and theranostics, such as active cargo delivery and microsurgical manipulations in hard-to-reach body sites. High-resolution imaging and control of cell-sized microrobots in the in vivo vascular system remains an unsolved challenge toward their clinical use. To overcome this limitation, we propose noninvasive real-time detection and tracking of circulating microrobots using optoacoustic imaging. We devised cell-sized nickel-based spherical Janus magnetic microrobots whose near-infrared optoacoustic signature is enhanced via gold conjugation. The 5-, 10-, and 20-μm-diameter microrobots are detected volumetrically both in bloodless ex vivo tissues and under real-life conditions with a strongly light-absorbing blood background. We further demonstrate real-time three-dimensional tracking and magnetic manipulation of the microrobots circulating in murine cerebral vasculature, thus paving the way toward effective and safe operation of cell-sized microrobots in challenging and clinically relevant intravascular environments.
Figures
References
-
- M. Sitti, Mobile Microrobotics (MIT Press, 2017).
-
- Ceylan H., Yasa I. C., Kilic U., Hu W., Sitti M., Translational prospects of untethered medical microrobots. Prog. Biomed. Eng. 1, 012002 (2019).
-
- Medina-Sánchez M., Xu H., Schmidt O. G., Micro- and nano-motors: The new generation of drug carriers. Ther. Deliv. 9, 303–316 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

