Tankyrase-mediated ADP-ribosylation is a regulator of TNF-induced death
- PMID: 35544574
- PMCID: PMC9094663
- DOI: 10.1126/sciadv.abh2332
Tankyrase-mediated ADP-ribosylation is a regulator of TNF-induced death
Abstract
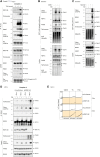
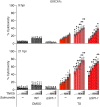
Tumor necrosis factor (TNF) is a key component of the innate immune response. Upon binding to its receptor, TNFR1, it promotes production of other cytokines via a membrane-bound complex 1 or induces cell death via a cytosolic complex 2. To understand how TNF-induced cell death is regulated, we performed mass spectrometry of complex 2 and identified tankyrase-1 as a native component that, upon a death stimulus, mediates complex 2 poly-ADP-ribosylation (PARylation). PARylation promotes recruitment of the E3 ligase RNF146, resulting in proteasomal degradation of complex 2, thereby limiting cell death. Expression of the ADP-ribose-binding/hydrolyzing severe acute respiratory syndrome coronavirus 2 macrodomain sensitizes cells to TNF-induced death via abolishing complex 2 PARylation. This suggests that disruption of ADP-ribosylation during an infection can prime a cell to retaliate with an inflammatory cell death.
Figures





References
-
- Silke J., The regulation of TNF signalling: What a tangled web we weave. Curr. Opin. Immunol. 23, 620–626 (2011). - PubMed
-
- Annibaldi A., Meier P., Checkpoints in TNF-induced cell death: Implications in inflammation and cancer. Trends Mol. Med. 24, 49–65 (2018). - PubMed
-
- Walczak H., TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol. Rev. 244, 9–28 (2011). - PubMed
-
- Silke J., Hartland E. L., Masters, marionettes and modulators: Intersection of pathogen virulence factors and mammalian death receptor signaling. Curr. Opin. Immunol. 25, 436–440 (2013). - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

