Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study
- PMID: 35544601
- PMCID: PMC9437712
- DOI: 10.1182/blood.2021015114
Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study
Abstract
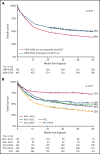
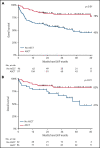
Patients aged <65 years with peripheral T-cell lymphoma (PTCL) are treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Although the addition of etoposide (CHOEP) and consolidation with autologous stem cell transplantation (ASCT) are preferred in some countries, randomized trials are lacking. This nationwide population-based study assessed the impact of etoposide and ASCT on overall survival (OS) among patients aged 18 to 64 years with stage II to IV anaplastic large-cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL), or PTCL not otherwise specified (NOS) diagnosed between 1989 and 2018 using the Netherlands Cancer Registry. Patients were categorized into 2 calendar periods, representing pre- and post-eras of etoposide and ASCT, respectively. A total of 1427 patients were identified (ALCL, 35%; AITL, 21%; and PTCL NOS, 44%). OS increased from 39% in the period from 1989 to 2009 to 49% in the period of 2009 to 2018 (P < .01). Five-year OS was superior for patients treated with CHOEP vs CHOP (64% and 44%, respectively; P < .01). When adjusted for subtype, International Prognostic Index score, and ASCT, the risk of mortality was similar between the 2 groups, except for patients with ALK+ ALCL, for whom the risk of mortality was 6.3 times higher when treated with CHOP vs CHOEP. Patients undergoing consolidation with ASCT had superior 5-year OS of 81% compared with 39% for patients not undergoing ASCT (P < .01), regardless of whether complete remission was achieved. In patients aged <65 years with advanced-stage ALK- ALCL, AITL, or PTCL, the use of ASCT consolidation, but not the addition of etoposide, was associated with improved OS.
© 2022 by The American Society of Hematology.
Figures



Comment in
-
To transplant or not to transplant: that is the question in PTCL.Blood. 2022 Sep 1;140(9):936-937. doi: 10.1182/blood.2022016489. Blood. 2022. PMID: 36048475 No abstract available.
References
-
- Stewart BW, Wild CP. World Cancer Report 2014. Lyon, France: IARC Press; 2014.
-
- Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124(10):1570-1577. - PubMed
-
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project . International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130. - PubMed
-
- Swerdlow SH, Campo E, Harris NL, et al. . Mature T- and NK-cell neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon, France: IARC Press; 2017:345-422.
-
- Petrich AM, Helenowski IB, Bryan LJ, Rozell SA, Galamaga R, Nabhan C. Factors predicting survival in peripheral T-cell lymphoma in the USA: a population-based analysis of 8802 patients in the modern era. Br J Haematol. 2015;168(5):708-718. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

