Innate immune cell response to host-parasite interaction in a human intestinal tissue microphysiological system
- PMID: 35544643
- PMCID: PMC9075809
- DOI: 10.1126/sciadv.abm8012
Innate immune cell response to host-parasite interaction in a human intestinal tissue microphysiological system
Abstract
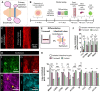
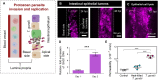
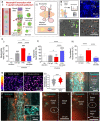
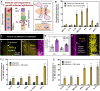
Protozoan parasites that infect humans are widespread and lead to varied clinical manifestations, including life-threatening illnesses in immunocompromised individuals. Animal models have provided insight into innate immunity against parasitic infections; however, species-specific differences and complexity of innate immune responses make translation to humans challenging. Thus, there is a need for in vitro systems that can elucidate mechanisms of immune control and parasite dissemination. We have developed a human microphysiological system of intestinal tissue to evaluate parasite-immune-specific interactions during infection, which integrates primary intestinal epithelial cells and immune cells to investigate the role of innate immune cells during epithelial infection by the protozoan parasite, Toxoplasma gondii, which affects billions of people worldwide. Our data indicate that epithelial infection by parasites stimulates a broad range of effector functions in neutrophils and natural killer cell-mediated cytokine production that play immunomodulatory roles, demonstrating the potential of our system for advancing the study of human-parasite interactions.
Figures


References
-
- Buzoni-Gatel D., Schulthess J., Menard L. C., Kasper L. H., Mucosal defences against orally acquired protozoan parasites, emphasis on Toxoplasma gondii infections. Cell. Microbiol. 8, 535–544 (2006). - PubMed
-
- Mestas J., Hughes C. C. W., Of mice and not men: Differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

