Implementation Strategies to Increase Clinical Trial Enrollment in a Community-Academic Partnership and Impact on Hispanic Representation: An Interrupted Time Series Analysis
- PMID: 35544650
- PMCID: PMC10166438
- DOI: 10.1200/OP.22.00037
Implementation Strategies to Increase Clinical Trial Enrollment in a Community-Academic Partnership and Impact on Hispanic Representation: An Interrupted Time Series Analysis
Abstract
Purpose: Community-academic partnerships have the potential to improve access to clinical trials for under-represented minority patients who more often receive cancer treatment in community settings. In 2017, the Memorial Sloan Kettering (MSK) Cancer Center began opening investigator-initiated clinical trials in radiation oncology in targeted community-based partner sites with a high potential to improve diverse population accrual. This study evaluates the effectiveness of a set of implementation strategies for increasing overall community-based enrollment and the resulting proportional enrollment of Hispanic patients on trials on the basis of availability in community-based partner sites.
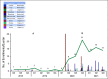
Methods: An interrupted time series analysis evaluating implementation strategies was conducted from April 2018 to September 2021. Descriptive analysis ofHispanic enrollment on investigator-initiated randomized therapeutic radiation trials open at community-based sites was compared with those open only at themain academic center.
Results: Overall, 84 patients were enrolled in clinical trials in the MSK Alliance, of which 48 (56%) identified as Hispanic. The quarterly patient enrollment pre- vs postimplementation increased from 1.39 (95% CI, -3.67 to 6.46) to 9.42 (95% CI, 2.05 to 16.78; P5 .017). In the investigator-initiated randomized therapeutic radiation trials open in the MSK Alliance, Hispanic representation was 11.5% and 35.9% in twometastatic trials and 14.2% in a proton versus photon trial. Inmatched trials open only at the main academic center, Hispanic representation was 5.6%, 6.0%, and 4.0%, respectively.
Conclusion: A combination of practice-level and physician-level strategies implemented at community-based partner sites was associated with increased clinical trial enrollment, which translated to improved Hispanic representation. This supports the role Q:2 of strategic community-academic partnerships in addressing disparities in clinical trial enrollment.
Conflict of interest statement
Figures

References
-
- National Institutes of Health . Policy and Compliance. National Institutes of Health; 2017. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research.https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines....
-
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials. JAMA. 2004;291:2720–2726. - PubMed
-
- Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: Review of the past 14 years. J Oncol Pract. 2018;14:e1–e10. - PubMed
-
- Bero EH, Rein LE, Banerjee A, et al. Characterization of underrepresented populations in modern era clinical trials involving radiation therapy. Pract Radiat Oncol. 2021;11:453–459. - PubMed
-
- Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care . In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, editors. Washington, DC: National Academies Press (US); 2003. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

