TRPC4 and GIRK channels underlie neuronal coding of firing patterns that reflect Gq/11-Gi/o coincidence signals of variable strengths
- PMID: 35544691
- PMCID: PMC9171772
- DOI: 10.1073/pnas.2120870119
TRPC4 and GIRK channels underlie neuronal coding of firing patterns that reflect Gq/11-Gi/o coincidence signals of variable strengths
Abstract
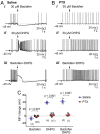
Transient receptor potential canonical 4 (TRPC4) is a receptor-operated cation channel codependent on both the Gq/11–phospholipase C signaling pathway and Gi/o proteins for activation. This makes TRPC4 an excellent coincidence sensor of neurotransmission through Gq/11- and Gi/o-coupled receptors. In whole-cell slice recordings of lateral septal neurons, TRPC4 mediates a strong depolarizing plateau that shuts down action potential firing, which may or may not be followed by a hyperpolarization that extends the firing pause to varying durations depending on the strength of Gi/o stimulation. We show that the depolarizing plateau is codependent on Gq/11-coupled group I metabotropic glutamate receptors and on Gi/o-coupled γ-aminobutyric acid type B receptors. The hyperpolarization is mediated by Gi/o activation of G protein–activated inwardly rectifying K+ (GIRK) channels. Moreover, the firing patterns, elicited by either electrical stimulation or receptor agonists, encode information about the relative strengths of Gq/11 and Gi/o inputs in the following fashion. Pure Gq/11 input produces weak depolarization accompanied by firing acceleration, whereas pure Gi/o input causes hyperpolarization that pauses firing. Although coincident Gq/11–Gi/o inputs also pause firing, the pause is preceded by a burst, and both the pause duration and firing recovery patterns reflect the relative strengths of Gq/11 versus Gi/o inputs. Computer simulations demonstrate that different combinations of TRPC4 and GIRK conductances are sufficient to produce the range of firing patterns observed experimentally. Thus, concurrent neurotransmission through the Gq/11 and Gi/o pathways is converted to discernible electrical responses by the joint actions of TRPC4 and GIRK for communication to downstream neurons.
Keywords: G proteins; TRP channels; coincidence detection; neuronal firing; neurotransmission.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
Multi-neurotransmitter regulation of neural firing via coincidence of parallel G-protein signals.Cell Calcium. 2022 Jul;105:102611. doi: 10.1016/j.ceca.2022.102611. Epub 2022 Jun 9. Cell Calcium. 2022. PMID: 35709660
-
Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels.Proc Natl Acad Sci U S A. 2016 Jan 26;113(4):1092-7. doi: 10.1073/pnas.1522294113. Epub 2016 Jan 11. Proc Natl Acad Sci U S A. 2016. PMID: 26755577 Free PMC article.
-
Intracellular acidification facilitates receptor-operated TRPC4 activation through PLCδ1 in a Ca2+ -dependent manner.J Physiol. 2020 Jul;598(13):2651-2667. doi: 10.1113/JP279658. Epub 2020 May 22. J Physiol. 2020. PMID: 32338378 Free PMC article.
-
Direct modulation of TRPC ion channels by Gα proteins.Front Physiol. 2024 Feb 7;15:1362987. doi: 10.3389/fphys.2024.1362987. eCollection 2024. Front Physiol. 2024. PMID: 38384797 Free PMC article. Review.
-
The Roles of Gβγ and Gα in Gating and Regulation of GIRK Channels.Int Rev Neurobiol. 2015;123:27-85. doi: 10.1016/bs.irn.2015.06.001. Epub 2015 Jul 26. Int Rev Neurobiol. 2015. PMID: 26422982 Review.
Cited by
-
Molecular Organization of Autonomic, Respiratory, and Spinally-Projecting Neurons in the Mouse Ventrolateral Medulla.J Neurosci. 2024 Jul 31;44(31):e2211232024. doi: 10.1523/JNEUROSCI.2211-23.2024. J Neurosci. 2024. PMID: 38918066 Free PMC article.
-
Cryo-EM structure of the heteromeric TRPC1/TRPC4 channel.Nat Struct Mol Biol. 2025 Feb;32(2):326-338. doi: 10.1038/s41594-024-01408-1. Epub 2024 Oct 30. Nat Struct Mol Biol. 2025. PMID: 39478185
-
Estradiol elicits distinct firing patterns in arcuate nucleus kisspeptin neurons of females through altering ion channel conductances.Elife. 2024 Dec 13;13:RP96691. doi: 10.7554/eLife.96691. Elife. 2024. PMID: 39671233 Free PMC article.
-
Estradiol elicits distinct firing patterns in arcuate nucleus kisspeptin neurons of females through altering ion channel conductances.bioRxiv [Preprint]. 2024 Sep 3:2024.02.20.581121. doi: 10.1101/2024.02.20.581121. bioRxiv. 2024. Update in: Elife. 2024 Dec 13;13:RP96691. doi: 10.7554/eLife.96691. PMID: 38915596 Free PMC article. Updated. Preprint.
-
Canonical transient receptor potential channels and hypothalamic control of homeostatic functions.J Neuroendocrinol. 2024 Oct;36(10):e13392. doi: 10.1111/jne.13392. Epub 2024 Apr 17. J Neuroendocrinol. 2024. PMID: 38631680 Review.
References
-
- Huang C. L., Feng S., Hilgemann D. W., Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature 391, 803–806 (1998). - PubMed
-
- Zhang H., He C., Yan X., Mirshahi T., Logothetis D. E., Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat. Cell Biol. 1, 183–188 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

