Negative Ultraselection of Patients With RAS/ BRAF Wild-Type, Microsatellite-Stable Metastatic Colorectal Cancer Receiving Anti-EGFR-Based Therapy
- PMID: 35544729
- PMCID: PMC9200389
- DOI: 10.1200/PO.22.00037
Negative Ultraselection of Patients With RAS/ BRAF Wild-Type, Microsatellite-Stable Metastatic Colorectal Cancer Receiving Anti-EGFR-Based Therapy
Erratum in
-
Erratum.JCO Precis Oncol. 2022 Jul;6:e2200356. doi: 10.1200/PO.22.00356. JCO Precis Oncol. 2022. PMID: 35849487 Free PMC article. No abstract available.
Abstract
Purpose: Several uncommon genomic alterations beyond RAS and BRAFV600E mutations drive primary resistance to anti-epidermal growth factor receptors (EGFRs) in metastatic colorectal cancer (mCRC). Our PRESSING panel (including PIK3CA exon 20/AKT1/PTEN mutations, ERBB2/MET amplifications, gene fusions, and microsatellite instability-high status) represented a paradigm of negative hyperselection with more precise tailoring of EGFR blockade. However, a modest proportion of hyperselected mCRC has intrinsic resistance potentially driven by even rarer genomic alterations.
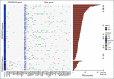
Materials and methods: A prospective data set at three Italian Academic Hospitals included 650 patients with mCRC with comprehensive genomic profiling by FoundationOne CDx and treated with anti-EGFRs. PRESSING2 panel alterations were selected on the basis of previous clinico-biologic studies and included NTRKs, ERBB3, NF1, MAP2K1/2/4, AKT2 pathogenic mutations; PTEN/NF1 loss; ERBB3, FGFR2, IGF1R, KRAS, ARAF, and AKT1-2 amplification; and EGFR rearrangements. These were collectively associated with outcomes in patients with hyperselected disease, ie, RAS/BRAF wild-type, PRESSING-negative, and microsatellite stable.
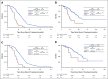
Results: Among 162 hyperselected patients, 24 (15%) had PRESSING2 alterations, which were mutually exclusive except in two samples and were numerically higher in right-sided versus left-sided cancers (28% v 13%; P = .149). Independently of sidedness and other factors, patients with PRESSING2-positive status had significantly worse progression-free survival and overall survival compared with PRESSING2-negative ones (median progression-free survival 6.4 v 12.8 months, adjusted hazard ratio 4.19 [95% CI, 2.58 to 6.79]; median overall survival: 22.6 v 49.9 months, adjusted hazard ratio 2.98 [95% CI, 1.49 to 5.96]). The combined analysis of primary tumor sidedness and PRESSING2 status allowed us to better stratify outcomes.
Conclusion: Negative ultraselection warrants further investigation with the aim of maximizing the benefit of EGFR blockade strategies in patients with RAS and BRAF wild-type, microsatellite stable mCRC.
Conflict of interest statement
Figures

References
-
- Yoshino T, Arnold D, Taniguchi H, et al. : Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 29:44-70, 2018 - PubMed
-
- Brule SY, Jonker DJ, Karapetis CS, et al. : Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer 51:1405-1414, 2015 - PubMed
-
- Dienstmann R, Vermeulen L, Guinney J, et al. : Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 17:79-92, 2017 - PubMed
-
- Bardelli A, Siena S: Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol 28:1254-1261, 2010 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

