Clinical and histopathological study of a hollow and posteriorly multiperforated polymethylmethacrylate implant in eviscerated rabbit eyes
- PMID: 35544934
- PMCID: PMC11826524
- DOI: 10.5935/0004-2749.20230064
Clinical and histopathological study of a hollow and posteriorly multiperforated polymethylmethacrylate implant in eviscerated rabbit eyes
Abstract
Purpose: The study aimed to evaluate the clinical and tissue response to a hollow polymethylmethacrylate orbital implant with a multiperforated posterior surface in an animal model after evisceration.
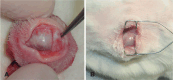
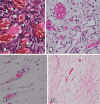
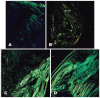
Methods: Sixteen New Zealand rabbits had their right eye eviscerated. All animals received a hollow polymethylmethacrylate implant 12 mm in diameter that is multiperforated in its posterior hemisphere. The animals were divided into four groups, and each one had the eye exenterated at 7, 30, 90, and 180 days post-evisceration. Clinical signs were assessed daily for 14 days post-evisceration and then every 7 days until 180 days. Inflammatory pattern, collagen structure, and degree of neovascularization generated with implant placement were analyzed with hematoxylin-eosin, picrosirius red, and immunohistochemistry staining.
Results: There were no signs of infection, conjunctival or scleral thinning, or implant exposure or extrusion in any animal during the study. On day 7, the new tissue migrated into the implant and formed a fibrovascular network through the posterior channels. Inflammatory response reduced over time, and no multinuclea-ted giant cells were found at any time.
Conclusion: Hollow polymethylmethacrylate orbital implants with a multiperforated posterior surface enable rapid integration with orbital tissues by fibrovascular ingrowth. We believe that this orbital implant model can be used in research on humans.
Objetivo: Avaliar a resposta tecidual e clínica a um implante orbitário de polimetilmetacrilato, oco e multiperfurado em sua porção posterior em modelo animal após evisceração.
Métodos: Dezesseis coelhos da raça Nova Zelândia foram submetidos à evisceração do globo ocular direito. Todos receberam implante oco de polimetilmetacrilato de 12 mm de diâmetro, multiperfurado em sua semiesfera posterior. O estudo foi dividido em avaliação clínica e histopatológica. A avaliação clínica foi diária até 14 dias pós-evisceração e, a cada sete dias, até completar 180 dias. Os animais foram divididos em grupos de quatro animais e cada um foi submetido à exenteração com 07, 30, 90 e 180 dias e depois à eutanásia. A análise histopatológica teve por fim caracterizar o padrão inflamatório, a estrutura do colágeno e o grau de neovascularização. Para isso, além da tradicional coloração pela hematoxilina-eosina, utilizou-se o corante Picrosirius Red (PSR) e imuno-histoquímica com o marcador CD 34.
Resultados: Não houve sinais de infecção, afinamento conjuntival ou escleral, exposição ou extrusão do implante em nenhum animal durante o estudo. Já no sétimo dia, o tecido neoformado migrou para dentro do implante formando uma rede fibrovascular através dos canais posteriores. A resposta inflamatória diminuiu ao longo do tempo avaliado e não foram encontradas células gigantes multinucleadas.
Conclusão: O implante analisado permite a sua integração aos tecidos orbitários pelo crescimento fibrovascular em seu interior. Os autores acreditam que este modelo de implante orbital pode fazer parte de testes com humanos.
Conflict of interest statement
Figures



References
-
- Oriá AP, Neto FA, Laus JL, Dos Santos LA, Piza ET, Brunelli AT, et al. Evaluation of a double-setting alpha-tricalcium phosphate cement in eviscerated rabbit eyes. Ophthal Plast Reconstr Surg. 2006;22(2):126–130. - PubMed
-
- Baino F, Perero S, Ferraris S, Miola M, Balagna C, Verné E, et al. Biomaterials for orbital implants and ocular prostheses: overview and future prospects. Acta Biomater. 2014;10(3):1064–1087. - PubMed
-
- Choi S, Lee SJ, Shin JH, Cheong Y, Lee HJ, Paek JH, et al. Ultrastructural investigation of intact orbital implant surfaces using atomic force microscopy. Scanning. 2011;33(4):211–221. - PubMed
-
- Sami D, Young S, Petersen R. Perspective on orbital enucleation implants. Surv Ophthalmol. 2007;52(3):244–265. - PubMed
-
- Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

