IL-1 Mediates Tissue-Specific Inflammation and Severe Respiratory Failure in COVID-19
- PMID: 35545011
- PMCID: PMC9801253
- DOI: 10.1159/000524560
IL-1 Mediates Tissue-Specific Inflammation and Severe Respiratory Failure in COVID-19
Abstract
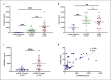
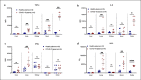
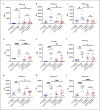
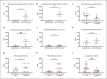
Acute respiratory distress syndrome (ARDS) in COVID-19 has been associated with catastrophic inflammation. We present measurements in humans and a new animal model implicating a role in danger-associated molecular patterns. Calprotectin (S100A8/A9) and high-mobility group box 1 (HMGB1) were measured in patients without/with ARDS, and admission calprotectin was associated with soluble urokinase plasminogen activator receptor (suPAR). An animal model was developed by intravenous injection of plasma from healthy or patients with COVID-19 ARDS into C57/BL6 mice once daily for 3 consecutive days. Mice were treated with one anti-S100A8/A9 antibody, the IL-1 receptor antagonist anakinra or vehicle, and Flo1-2a anti-murine anti-IL-1α monoclonal antibody or the specific antihuman IL-1α antibody XB2001 or isotype controls. Cytokines and myeloperoxidase (MPO) were measured in tissues. Calprotectin, but not HMGB1, was elevated in ARDS. Higher suPAR indicated higher calprotectin. Animal challenge with COVID-19 plasma led to inflammatory reactions in murine lung and intestines as evidenced by increased levels of TNFα, IL-6, IFNγ, and MPO. Lung inflammation was attenuated with anti-S100A8/A9 pre-treatment. Anakinra treatment restored these levels. Similar decrease was found in mice treated with Flo1-2a but not with XB2001. Circulating alarmins, specifically calprotectin, of critically ill COVID-19 patients induces tissue-specific inflammatory responses through an IL-1-mediated mechanism. This could be attenuated through inhibition of IL-1 receptor or of IL-1α.
Trial registration: ClinicalTrials.gov NCT04357366.
Keywords: Acute respiratory distress syndrome; Calprotectin; Coronavirus diseases 2019; Interleukin-1; Interleukin-6; Severe respiratory failure; Soluble urokinase plasminogen activator receptor; Tumor necrosis factor.
© 2022 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
J. Eugen-Olsen is a cofounder, shareholder, and CSO of ViroGates A7S, Denmark, and is named inventor on patents on suPAR owned by Copenhagen University Hospital Hvidovre, Denmark. He is granted by the Horizon 2020 European Grant RISCinCOVID. J. Simard is the CEO and founder of XBiotech. M.G. Netea is a scientific founder of TTxD and received research grants from GSK and ViiV Healthcare. E.J. Giamarellos-Bourboulis has received honoraria from AbbVie USA, Abbott CH, InflaRx GmbH, MSD Greece, XBiotech Inc., and Angelini Italy; independent educational grants from AbbVie, Abbott, Astellas Pharma Europe, Axis Shield, bioMérieux Inc, InflaRx GmbH, Sobi, and XBiotech Inc.; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon 2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis).
Figures

References
-
- Aeffner F, Bolon B, Davis IC. Mouse models of acute respiratory distress syndrome: a review of analytical approaches, pathologic features, and common measurements. Toxicol Pathol. 2015;43:1074–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

