Clinical activity of PD-1 inhibition in the treatment of locally advanced or metastatic basal cell carcinoma
- PMID: 35545318
- PMCID: PMC9096532
- DOI: 10.1136/jitc-2022-004839
Clinical activity of PD-1 inhibition in the treatment of locally advanced or metastatic basal cell carcinoma
Abstract
Background: Basal cell carcinoma (BCC) is the most common malignancy worldwide, yet the management of patients with advanced or metastatic disease is challenging, with limited treatment options. Recently, programmed death receptor 1 (PD-1) inhibition has demonstrated activity in BCC after prior Hedgehog inhibitor treatment.
Methods: We conducted a multicenter, retrospective analysis of BCC patients treated with PD-1 inhibitor therapy. We examined the efficacy and safety of PD-1 therapy, as well as clinical and pathological variables in association with outcomes. Progression-free survival (PFS), overall survival (OS) and duration of response (DOR) were calculated using Kaplan-Meier methodology. Toxicity was graded per Common Terminology Criteria for Adverse Events V.5.0.
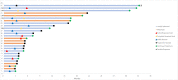
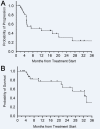
Results: A total of 29 patients with BCC who were treated with PD-1 inhibition were included for analysis, including 20 (69.0%) with locally advanced and 9 (31.0%) with metastatic disease. The objective response rate was 31.0%, with five partial responses (17.2%), and four complete responses (13.8%). Nine patients had stable disease (31.0%), with a disease control rate of 62.1%. The median DOR was not reached. Median PFS was 12.2 months (95% CI 0.0 to 27.4). Median OS was 32.4 months (95% CI 18.1 to 46.7). Two patients (6.9%) developed grade 3 or higher toxicity, while four patients (13.8%) discontinued PD-1 inhibition because of toxicity. Higher platelets (p=0.022) and any grade toxicity (p=0.024) were significantly associated with disease control rate.
Conclusions: The clinical efficacy of PD-1 inhibition among patients with advanced or metastatic BCC in this real-world cohort were comparable to published trial data. Further investigation of PD-1 inhibition is needed to define its optimal role for patients with this disease.
Keywords: immunotherapy; programmed cell death 1 receptor; skin neoplasms.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GKI reports having consulting/advisory board roles with Boehringer-Ingelheim, Novartis, BMS, Castle Biosciences, Regeneron, Sanofi; receiving research support from Regeneron, Array, Idera, Roche/Genentech, Replimune, Xencor, InstilBio, and having speaker roles for Merck. DYR reports having consulting/advisory board roles with Castle Biosciences. JCH reports having consulting/advisory board roles with Regeneron. All other authors have nothing to disclose.
Figures
Similar articles
-
Final analysis of phase II results with cemiplimab in metastatic basal cell carcinoma after hedgehog pathway inhibitors.Ann Oncol. 2024 Feb;35(2):221-228. doi: 10.1016/j.annonc.2023.10.123. Epub 2023 Dec 9. Ann Oncol. 2024. PMID: 38072158 Clinical Trial.
-
Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial.Lancet Oncol. 2021 Jun;22(6):848-857. doi: 10.1016/S1470-2045(21)00126-1. Epub 2021 May 14. Lancet Oncol. 2021. PMID: 34000246 Clinical Trial.
-
Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study.BMC Cancer. 2017 May 16;17(1):332. doi: 10.1186/s12885-017-3286-5. BMC Cancer. 2017. PMID: 28511673 Free PMC article. Clinical Trial.
-
A comprehensive review of the role of the hedgehog pathway and vismodegib in the management of basal cell carcinoma.Curr Med Res Opin. 2015 Apr;31(4):743-56. doi: 10.1185/03007995.2015.1018988. Epub 2015 Mar 17. Curr Med Res Opin. 2015. PMID: 25690490 Review.
-
Vismodegib: in locally advanced or metastatic basal cell carcinoma.Drugs. 2012 Jul 30;72(11):1535-41. doi: 10.2165/11209590-000000000-00000. Drugs. 2012. PMID: 22788238 Review.
Cited by
-
Immunotherapy for locally advanced and metastatic basal cell carcinoma: a narrative review.Transl Cancer Res. 2024 Nov 30;13(11):6565-6575. doi: 10.21037/tcr-24-742. Epub 2024 Nov 6. Transl Cancer Res. 2024. PMID: 39697716 Free PMC article. Review.
-
Basosquamous Carcinoma: Comprehensive Clinical and Histopathological Aspects, Novel Imaging Tools, and Therapeutic Approaches.Cells. 2023 Nov 30;12(23):2737. doi: 10.3390/cells12232737. Cells. 2023. PMID: 38067165 Free PMC article. Review.
-
Hedgehog Pathway and Programmed Cell Death Protein-1 Inhibitors for Advanced Basal Cell Carcinoma.Case Rep Dermatol. 2024 Jun 27;16(1):173-180. doi: 10.1159/000539592. eCollection 2024 Jan-Dec. Case Rep Dermatol. 2024. PMID: 39015399 Free PMC article.
-
The Unexpected Guest: Basal Cell Carcinoma on a Covered Site.Cureus. 2025 Jan 30;17(1):e78259. doi: 10.7759/cureus.78259. eCollection 2025 Jan. Cureus. 2025. PMID: 40026985 Free PMC article.
-
Multicenter proteome-wide Mendelian randomization study identifies causal plasma proteins in melanoma and non-melanoma skin cancers.Commun Biol. 2024 Jul 13;7(1):857. doi: 10.1038/s42003-024-06538-2. Commun Biol. 2024. PMID: 39003418 Free PMC article.
References
-
- Szeimies RM, Ibbotson S, Murrell DF, et al. . A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8-20 mm), with a 12-month follow-up. J Eur Acad Dermatol Venereol 2008;22:1302–11. 10.1111/j.1468-3083.2008.02803.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
