STAndardised DIagnostic Assessment for children and young people with emotional difficulties (STADIA): protocol for a multicentre randomised controlled trial
- PMID: 35545388
- PMCID: PMC9096530
- DOI: 10.1136/bmjopen-2021-053043
STAndardised DIagnostic Assessment for children and young people with emotional difficulties (STADIA): protocol for a multicentre randomised controlled trial
Abstract
Introduction: Emotional disorders (such as anxiety and depression) are associated with considerable distress and impairment in day-to-day function for affected children and young people and for their families. Effective evidence-based interventions are available but require appropriate identification of difficulties to enable timely access to services. Standardised diagnostic assessment (SDA) tools may aid in the detection of emotional disorders, but there is limited evidence on the utility of SDA tools in routine care and equipoise among professionals about their clinical value.
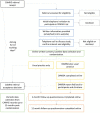
Methods and analysis: A multicentre, two-arm, parallel group randomised controlled trial, with embedded qualitative and health economic components. Participants will be randomised in a 1:1 ratio to either the Development and Well-Being Assessment SDA tool as an adjunct to usual clinical care, or usual care only. A total of 1210 participants (children and young people referred to outpatient, specialist Child and Adolescent Mental Health Services with emotional difficulties and their parent/carers) will be recruited from at least 6 sites in England. The primary outcome is a clinician-made diagnosis about the presence of an emotional disorder within 12 months of randomisation. Secondary outcomes include referral acceptance, diagnosis and treatment of emotional disorders, symptoms of emotional difficulties and comorbid disorders and associated functional impairment.
Ethics and dissemination: The study received favourable opinion from the South Birmingham Research Ethics Committee (Ref. 19/WM/0133). Results of this trial will be reported to the funder and published in full in the Health Technology Assessment (HTA) Journal series and also submitted for publication in a peer reviewed journal.
Trial registration number: ISRCTN15748675; Pre-results.
Keywords: child & adolescent psychiatry; depression & mood disorders; health economics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Sadler K, Vizard T, Ford T. Mental health of children and young people in England 2017 2018. https://digital.nhs.uk/data-and-information/publications/statistical/men... - PubMed
-
- Goodyer IM, Reynolds S, Barrett B, et al. Cognitive–behavioural therapy and short-term psychoanalytic psychotherapy versus brief psychosocial intervention in adolescents with unipolar major depression (IMPACT): a multicentre, pragmatic, observer-blind, randomised controlled trial. Health Technol Assess 2017;21:hta21120 10.3310/hta21120 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
