Knockout of farnesoid X receptor gene aggravates cisplatin-induced kidney injury
- PMID: 35545407
- PMCID: PMC10930519
- DOI: 10.11817/j.issn.1672-7347.2022.210423
Knockout of farnesoid X receptor gene aggravates cisplatin-induced kidney injury
Abstract
Objectives: Farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily of ligand activated transcription factors and belongs to bile acid receptor. Studies have shown that the expression of FXR in renal tissue can reduce renal injury via regulation of glucose and lipid metabolism, inhibition of inflammatory response, reduction of oxidative stress and renal fibrosis. However, it is unclear whether FXR is involved in autophagy in renal diseases. This study aims to investigate the role of FXR in cisplatin-induced acute renal injury and whether its mechanism is related to autophagy regulation.
Methods: Twelve male WT or FXR-KO mice at 12 weeks were randomly divided into a WT group, a WT+cisplatin group, a FXR-KO group, and a FXR-KO+cisplatin group, with 6 mice in each group. The WT+cisplatin group and the FXR-KO+cisplatin group were intraperitoneally injected with cisplatin (20 mg/kg), and the WT group and the FXR-KO group were intraperitoneally injected with equal volume of cisplatin solvent. Seventy-two hours later, the mice were killed and blood and renal tissue samples were collected. The levels of SCr and BUN were detected by immunoturbidimetry. After the staining, the pathological changes of renal tissue were observed under optical microscope. The protein levels of LC3 and p62 were detected by Western blotting and immunohistochemistry. The clearance of damaged mitochondria and the accumulation of lysosomal substrate were observed under electron microscope. The apoptosis of renal tubular epithelial cells was detected by TUNEL.
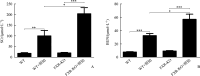
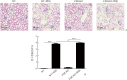
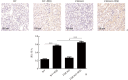
Results: Compared with the WT group or the FXR-KO group, both SCr and BUN levels in the WT+cisplatin group or the FXR-KO+cisplatin group were significantly increased (P<0.01 or P<0.001), and SCr and BUN levels in the FXR-KO+cisplatin group were significantly higher than those in the WT+cisplatin group (both P<0.05). Under the light microscope, there were no obvious pathological changes in the renal tissue of mice in the WT group and the FXR-KO group. Both the WT+cisplatin group and the FXR-KO+cisplatin group had vacuolar or granular degeneration of renal tubular epithelial cells, flat cells, lumen expansion, brush edge falling off, and even exposed basement membrane and tubular formation. The scores of renal tubular injury in the WT+cisplatin group and the FXR-KO+cisplatin group were significantly higher than those in the WT group and the FXR-KO group, respectively (both P<0.001), and the score in the FXR-KO+cisplatin group was significantly higher than that in the WT+cisplatin group (P<0.05). Under the transmission electron microscope, the mitochondria of mouse tubular epithelial cell in the WT+cisplatin group and the FXR-KO+cisplatin group was swollen, round, vacuolated, cristae broken or disappeared; the lysosome was uneven and high-density clumps, and the change was more obvious in the FXR-KO+cisplatin group. Western blotting showed that the ratio of LC3-II to LC3-I was decreased and the expression of p62 was increased in the WT+cisplatin group compared with the WT group and the FXR-KO+cisplatin group compared with FXR-KO group (P<0.05 or P<0.01); compared with the FXR-KO group, the ratio of LC3-II to LC3-I was decreased and the expression of p62 was increased significantly in the FXR-KO+cisplatin group (both P<0.05). Immunohistochemistry results showed that the expression of total LC3 and p62 in renal cortex of the WT+cisplatin group and the FXR-KO+cisplatin group was increased significantly, especially in the FXR-KO+cisplatin group. TUNEL results showed that the mice in the WT group and the FXR-KO group had negative staining or only a few apoptotic tubular epithelial cells, and the number of apoptotic cells in the WT+cisplatin group and the FXR-KO+cisplatin group were increased. The apoptosis rates of renal tubular epithelial cells in the WT+cisplatin group and the FXR-KO+cisplatin group were significantly higher than those in the WT group and the FXR-KO group, respectively (both P<0.001), and the apoptosis rate in the FXR-KO+cisplatin group was significantly higher than that in the WT+cisplatin group (P<0.05).
Conclusions: Knockout of FXR gene aggravates cisplatin induced acute renal injury, and its mechanism may be related to inhibiting autophagy and promoting apoptosis.
目的: 法尼酯X受体(farnesoid X receptor,FXR)是配体激活转录因子核受体超家族成员,属于胆汁酸受体。FXR在肾组织中表达,可通过调节糖脂代谢、抑制炎症反应、拮抗氧化应激及肾纤维化等机制减轻肾损伤。但FXR是否参与肾脏疾病中的自噬尚不明确。本研究旨在探讨FXR在顺铂所致急性肾损伤中的作用,并探讨其机制是否与调控自噬相关。方法: 选取12周雄性野生型(wild type,WT)或FXR基因敲除(knockout of FXR,FXR-KO)小鼠各12只,随机分为WT组、WT+顺铂组、FXR-KO组、FXR-KO+顺铂组,每组6只,WT+顺铂组和FXR-KO+顺铂组予腹腔注射顺铂(20 mg/kg),WT组和FXR-KO组予腹腔注射等体积的顺铂溶剂。72 h后处死小鼠,留取血液和肾组织标本。采用免疫比浊法检测血清BUN和SCr水平,HE染色后在光学显微镜下观察肾组织病理改变,采用蛋白质印迹法和免疫组织化学法检测LC3、p62的蛋白质表达水平,在电子显微镜下观察受损线粒体清除和溶酶体底物聚集的情况,采用TUNEL检测肾小管上皮细胞凋亡的情况。结果: WT+顺铂组相较于WT组,FXR-KO+顺铂组相较于FXR-KO组,小鼠SCr和BUN均明显升高(P<0.01或P<0.001),且FXR-KO+顺铂组小鼠SCr和BUN明显高于WT+顺铂组(均P<0.05)。在光学显微镜下可见WT组和FXR-KO组小鼠肾组织无明显病理改变,WT+顺铂组和FXR-KO+顺铂组小鼠均存在肾小管上皮细胞空泡样或颗粒样变性,细胞扁平,管腔扩张,刷状缘脱落,甚至出现基底膜裸露,管型形成。WT+顺铂组相较于WT组,FXR-KO+顺铂组相较于FXR-KO组,小鼠肾小管损伤评分均明显升高(均P<0.001),且FXR-KO+顺铂组评分明显高于WT+顺铂组(P<0.05)。在透射电镜下可见WT+顺铂组和FXR-KO+顺铂组小鼠小管上皮细胞线粒体肿胀变圆、空泡化、嵴断裂或消失,溶酶体呈不均匀、高密度团块状,且以FXR-KO+顺铂组改变更为明显。蛋白质印迹法结果显示:WT+顺铂组相较于WT组,FXR-KO+顺铂组相较于FXR-KO组,小鼠肾皮质LC3-II/LC3-I比值下降,p62表达增加(P<0.05或P<0.01);且相较于FXR-KO组,FXR-KO+顺铂组小鼠LC3-II/LC3-I比值下降,p62表达增加更为明显(均P<0.05)。免疫组织化学结果显示:WT+顺铂组和FXR-KO+顺铂组小鼠肾皮质总LC3和p62表达均明显增加,且以FXR-KO+顺铂组增加更为显著。TUNEL结果显示:WT组和FXR-KO组小鼠染色阴性或仅见数个小管上皮细胞凋亡,WT+顺铂组和FXR-KO+顺铂组凋亡细胞数量均增多;WT+顺铂组相较于WT组,FXR-KO+顺铂组相较于FXR-KO组,肾小管上皮细胞凋亡率明显增加(均P<0.001),且FXR-KO+顺铂组凋亡率明显高于WT+顺铂组(P<0.05)。结论: FXR基因敲除加重顺铂所致急性肾损伤,其机制可能与抑制自噬和促进凋亡有关。.
Keywords: acute renal injury; apoptosis; autophagy; cisplatin; farnesoid X receptor; gene knockout.
Conflict of interest statement
作者声称无任何利益冲突。
Figures


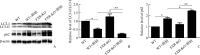

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

