Responses to Song Playback Differ in Sleeping versus Anesthetized Songbirds
- PMID: 35545423
- PMCID: PMC9131720
- DOI: 10.1523/ENEURO.0015-22.2022
Responses to Song Playback Differ in Sleeping versus Anesthetized Songbirds
Abstract
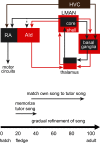
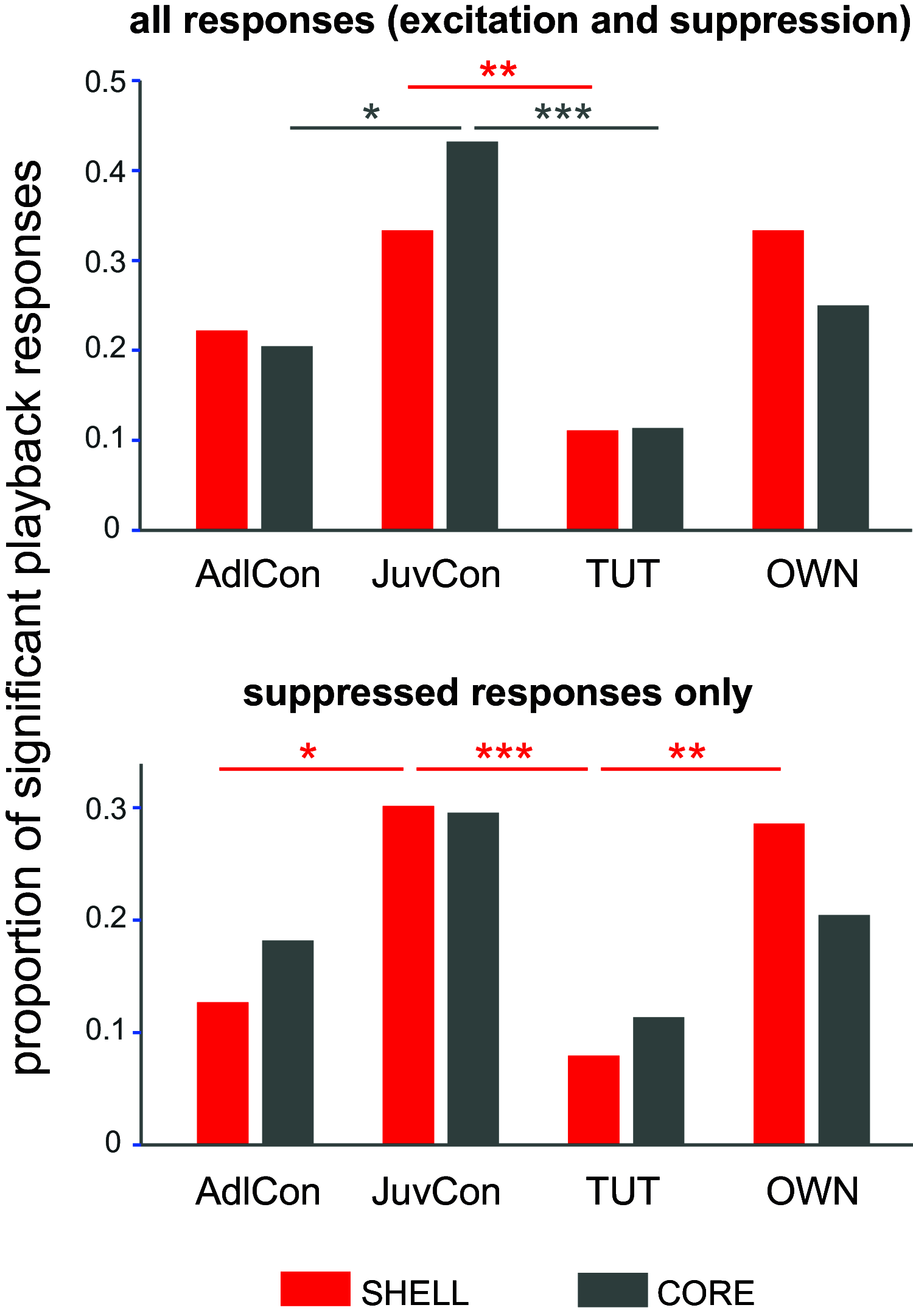
Vocal learning in songbirds is mediated by a highly localized system of interconnected forebrain regions, including recurrent loops that traverse the cortex, basal ganglia, and thalamus. This brain-behavior system provides a powerful model for elucidating mechanisms of vocal learning, with implications for learning speech in human infants, as well as for advancing our understanding of skill learning in general. A long history of experiments in this area has tested neural responses to playback of different song stimuli in anesthetized birds at different stages of vocal development. These studies have demonstrated selectivity for different song types that provide neural signatures of learning. In contrast to the ease of obtaining responses to song playback in anesthetized birds, song-evoked responses in awake birds are greatly reduced or absent, indicating that behavioral state is an important determinant of neural responsivity. Song-evoked responses can be elicited during sleep as well as anesthesia, and the selectivity of responses to song playback in adult birds is highly similar between anesthetized and sleeping states, encouraging the idea that anesthesia and sleep are similar. In contrast to that idea, we report evidence that cortical responses to song playback in juvenile zebra finches (Taeniopygia guttata) differ greatly between sleep and urethane anesthesia. This finding indicates that behavioral states differ in sleep versus anesthesia and raises questions about relationships between developmental changes in sleep activity, selectivity for different song types, and the neural substrate for vocal learning.
Keywords: anesthesia; sensory gating; sleep; songbird; spiking activity; vocal learning.
Copyright © 2022 Bottjer et al.
Figures



Similar articles
-
Differential developmental changes in cortical representations of auditory-vocal stimuli in songbirds.J Neurophysiol. 2019 Feb 1;121(2):530-548. doi: 10.1152/jn.00714.2018. Epub 2018 Dec 12. J Neurophysiol. 2019. PMID: 30540540 Free PMC article.
-
Sleep and sensorimotor integration during early vocal learning in a songbird.Nature. 2009 Mar 5;458(7234):73-7. doi: 10.1038/nature07615. Epub 2008 Dec 14. Nature. 2009. PMID: 19079238 Free PMC article.
-
Neural processing of auditory feedback during vocal practice in a songbird.Nature. 2009 Jan 8;457(7226):187-90. doi: 10.1038/nature07467. Epub 2008 Nov 12. Nature. 2009. PMID: 19005471
-
Cellular, circuit, and synaptic mechanisms in song learning.Ann N Y Acad Sci. 2004 Jun;1016:495-523. doi: 10.1196/annals.1298.035. Ann N Y Acad Sci. 2004. PMID: 15313792 Review.
-
Age and experience affect the recruitment of new neurons to the song system of zebra finches during the sensitive period for song learning: ditto for vocal learning in humans?Ann N Y Acad Sci. 2004 Jun;1021:404-9. doi: 10.1196/annals.1308.049. Ann N Y Acad Sci. 2004. PMID: 15251918 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
