Existence of a Long-Range Caudo-Rostral Sensory Influence in Terrestrial Locomotion
- PMID: 35545434
- PMCID: PMC9188425
- DOI: 10.1523/JNEUROSCI.2290-20.2022
Existence of a Long-Range Caudo-Rostral Sensory Influence in Terrestrial Locomotion
Abstract
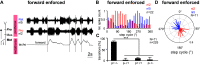
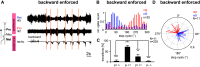
In multisegmented locomotion, coordination of all appendages is crucial for the generation of a proper motor output. In running for example, leg coordination is mainly based on the central interaction of rhythm generating networks, called central pattern generators (CPGs). In slower forms of locomotion, however, sensory feedback, which originates from sensory organs that detect changes in position, velocity and load of the legs' segments, has been shown to play a more crucial role. How exactly sensory feedback influences the activity of the CPGs to establish functional neuronal connectivity is not yet fully understood. Using the female stick insect Carausius morosus, we show for the first time that a long-range caudo-rostral sensory connection exists and highlight that load as sensory signal is sufficient to entrain rhythmic motoneuron (MN) activity in the most rostral segment. So far, mainly rostro-caudal influencing pathways have been investigated where the strength of activation, expressed by the MN activity in the thoracic ganglia, decreases with the distance from the stepping leg to these ganglia. Here, we activated CPGs, producing rhythmic neuronal activity in the thoracic ganglia by using the muscarinic agonist pilocarpine and enforced the stepping of a single, remaining leg. This enabled us to study sensory influences on the CPGs' oscillatory activity. Using this approach, we show that, in contrast to the distance-dependent activation of the protractor-retractor CPGs in different thoracic ganglia, there is no such dependence for the entrainment of the rhythmic activity of active protractor-retractor CPG networks by individual stepping legs.SIGNIFICANCE STATEMENT We show for the first time that sensory information is transferred not only to the immediate adjacent segmental ganglia but also to those farther away, indicating the existence of a long-range caudo-rostral sensory influence. This influence is dependent on stepping direction but independent of whether the leg is actively or passively moved. We suggest that the sensory information comes from unspecific load signals sensed by cuticle mechanoreceptors (campaniform sensilla) of a leg. Our results provide a neuronal basis for the long-established behavioral rules of insect leg coordination. We thus provide a breakthrough in understanding the neuronal networks underlying multilegged locomotion and open new vistas into the neuronal functional connectivity of multisegmented locomotion systems across the animal kingdom.
Keywords: CPG; entrainment; inter-segmental coordination; locomotion; six-legged walking.
Copyright © 2022 the authors.
Figures





Similar articles
-
Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system.J Neurosci. 2009 Mar 4;29(9):2972-83. doi: 10.1523/JNEUROSCI.3155-08.2009. J Neurosci. 2009. PMID: 19261892 Free PMC article.
-
Intra- and intersegmental influences among central pattern generating networks in the walking system of the stick insect.J Neurophysiol. 2017 Oct 1;118(4):2296-2310. doi: 10.1152/jn.00321.2017. Epub 2017 Jul 19. J Neurophysiol. 2017. PMID: 28724783 Free PMC article.
-
A mathematical modeling study of inter-segmental coordination during stick insect walking.J Comput Neurosci. 2011 Apr;30(2):255-78. doi: 10.1007/s10827-010-0254-3. Epub 2010 Jun 22. J Comput Neurosci. 2011. PMID: 20567889
-
Central pattern generating networks in insect locomotion.Dev Neurobiol. 2020 Jan;80(1-2):16-30. doi: 10.1002/dneu.22738. Epub 2020 Mar 23. Dev Neurobiol. 2020. PMID: 32128970 Review.
-
Six-legged walking in insects: how CPGs, peripheral feedback, and descending signals generate coordinated and adaptive motor rhythms.J Neurophysiol. 2018 Feb 1;119(2):459-475. doi: 10.1152/jn.00658.2017. Epub 2017 Oct 25. J Neurophysiol. 2018. PMID: 29070634 Review.
Cited by
-
Mechanosensory Control of Locomotion in Animals and Robots: Moving Forward.Integr Comp Biol. 2023 Aug 23;63(2):450-463. doi: 10.1093/icb/icad057. Integr Comp Biol. 2023. PMID: 37279901 Free PMC article. Review.
References
-
- Bässler U (1977) Sense organs in the femur of the stick insect and their relevance to the control of position of the femur-tibia-joint. J Comp Physiol 121:99–113.
-
- Batschelet E (1981) Circular statistics in biology. San Diego: Academic Press.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
