Simulated Attack Reveals How Lesions Affect Network Properties in Poststroke Aphasia
- PMID: 35545436
- PMCID: PMC9188386
- DOI: 10.1523/JNEUROSCI.1163-21.2022
Simulated Attack Reveals How Lesions Affect Network Properties in Poststroke Aphasia
Abstract
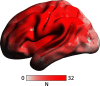
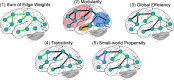
Aphasia is a prevalent cognitive syndrome caused by stroke. The rarity of premorbid imaging and heterogeneity of lesion obscures the links between the local effects of the lesion, global anatomic network organization, and aphasia symptoms. We applied a simulated attack approach in humans to examine the effects of 39 stroke lesions (16 females) on anatomic network topology by simulating their effects in a control sample of 36 healthy (15 females) brain networks. We focused on measures of global network organization thought to support overall brain function and resilience in the whole brain and within the left hemisphere. After removing lesion volume from the network topology measures and behavioral scores [the Western Aphasia Battery Aphasia Quotient (WAB-AQ), four behavioral factor scores obtained from a neuropsychological battery, and a factor sum], we compared the behavioral variance accounted for by simulated poststroke connectomes to that observed in the randomly permuted data. Global measures of anatomic network topology in the whole brain and left hemisphere accounted for 10% variance or more of the WAB-AQ and the lexical factor score beyond lesion volume and null permutations. Streamline networks provided more reliable point estimates than FA networks. Edge weights and network efficiency were weighted most highly in predicting the WAB-AQ for FA networks. Overall, our results suggest that global network measures provide modest statistical value beyond lesion volume when predicting overall aphasia severity, but less value in predicting specific behaviors. Variability in estimates could be induced by premorbid ability, deafferentation and diaschisis, and neuroplasticity following stroke.SIGNIFICANCE STATEMENT Poststroke, the remaining neuroanatomy maintains cognition and supports recovery. However, studies often use small, cross-sectional samples that cannot fully model the interactions between lesions and other variables that affect networks in stroke. Alternate methods are required to account for these effects. "Simulated attack" models are computational approaches that apply virtual damage to the brain and measure their putative consequences. Using a simulated attack model, we estimated how simulated damage to anatomic networks could account for language performance. Overall, our results reveal that global network measures can provide modest statistical value predicting overall aphasia severity, but less value in predicting specific behaviors. These findings suggest that more theoretically precise network models could be necessary to robustly predict individual outcomes in aphasia.
Keywords: WAB; aphasia; network; neuroimaging; simulated attack; stroke.
Copyright © 2022 the authors.
Figures





Similar articles
-
Multivariate Connectome-Based Symptom Mapping in Post-Stroke Patients: Networks Supporting Language and Speech.J Neurosci. 2016 Jun 22;36(25):6668-79. doi: 10.1523/JNEUROSCI.4396-15.2016. J Neurosci. 2016. PMID: 27335399 Free PMC article.
-
Language Recovery after Brain Injury: A Structural Network Control Theory Study.J Neurosci. 2022 Jan 26;42(4):657-669. doi: 10.1523/JNEUROSCI.1096-21.2021. Epub 2021 Dec 6. J Neurosci. 2022. PMID: 34872927 Free PMC article. Clinical Trial.
-
Advanced Brain Age and Chronic Poststroke Aphasia Severity.Neurology. 2023 Mar 14;100(11):e1166-e1176. doi: 10.1212/WNL.0000000000201693. Epub 2022 Dec 16. Neurology. 2023. PMID: 36526425 Free PMC article.
-
Functional MRI evidence for reorganization of language networks after stroke.Handb Clin Neurol. 2022;185:131-150. doi: 10.1016/B978-0-12-823384-9.00007-4. Handb Clin Neurol. 2022. PMID: 35078595 Review.
-
Imaging effects related to language improvements by rTMS.Restor Neurol Neurosci. 2016 Apr 11;34(4):531-6. doi: 10.3233/RNN-150631. Restor Neurol Neurosci. 2016. PMID: 27080074 Review.
Cited by
-
The evolution of whole-brain turbulent dynamics during recovery from traumatic brain injury.Netw Neurosci. 2024 Apr 1;8(1):158-177. doi: 10.1162/netn_a_00346. eCollection 2024. Netw Neurosci. 2024. PMID: 38562284 Free PMC article.
-
Turbulent dynamics and whole-brain modeling: toward new clinical applications for traumatic brain injury.Front Neuroinform. 2024 Mar 25;18:1382372. doi: 10.3389/fninf.2024.1382372. eCollection 2024. Front Neuroinform. 2024. PMID: 38590709 Free PMC article. Review.
-
Lesion-Induced Changes to the Network Controllability of the Right Pars Triangularis in Aphasia.Neurobiol Lang (Camb). 2025 Sep 2;6:nol.a.11. doi: 10.1162/nol.a.11. eCollection 2025. Neurobiol Lang (Camb). 2025. PMID: 40905000 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
