Copper-catalysed asymmetric reductive cross-coupling of prochiral alkenes
- PMID: 35545634
- PMCID: PMC9095606
- DOI: 10.1038/s41467-022-30286-8
Copper-catalysed asymmetric reductive cross-coupling of prochiral alkenes
Abstract
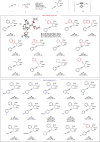
Asymmetric construction of C(sp3)-C(sp3) bond with good stereocontrol of the two connecting carbon centres retaining all carbon or hydrogen substituents is a challenging target in transition metal catalysis. Transition metal-catalysed reductive coupling of unsaturated π-substrates is considered as a potent tool to expediently develop the molecular complexity with high atom efficiency. However, such an asymmetric and intermolecular process has yet to be developed fully. Herein, we report an efficient strategy to reductively couple two prochiral conjugate alkenes using a copper-catalysed tandem protocol in the presence of diboron. Notably, this transformation incorporates a wide range of terminal and internal enynes as coupling partners and facilitates highly diastereo- and enantioselective synthesis of organoboron derivatives with multiple adjacent stereocentres in a single operation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Copper-Catalyzed Radical Relay for Asymmetric Radical Transformations.Acc Chem Res. 2018 Sep 18;51(9):2036-2046. doi: 10.1021/acs.accounts.8b00265. Epub 2018 Sep 5. Acc Chem Res. 2018. PMID: 30183262
-
Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)-C(sp) cross-coupling of tertiary electrophiles with alkynes.Nat Chem. 2022 Aug;14(8):949-957. doi: 10.1038/s41557-022-00954-9. Epub 2022 May 26. Nat Chem. 2022. PMID: 35618768
-
A direct approach to amines with remote stereocentres by enantioselective CuH-catalysed reductive relay hydroamination.Nat Chem. 2016 Feb;8(2):144-50. doi: 10.1038/nchem.2418. Epub 2016 Jan 4. Nat Chem. 2016. PMID: 26791897 Free PMC article.
-
Recent advances in transition-metal-catalysed asymmetric coupling reactions with light intervention.Chem Soc Rev. 2021 Nov 15;50(22):12808-12827. doi: 10.1039/d1cs00210d. Chem Soc Rev. 2021. PMID: 34652345 Review.
-
Progress in copper-catalysed/mediated intramolecular dehydrogenative coupling.Org Biomol Chem. 2023 Jan 4;21(2):237-251. doi: 10.1039/d2ob01796b. Org Biomol Chem. 2023. PMID: 36448561 Review.
Cited by
-
The potential of converting carbon dioxide to food compounds via asymmetric catalysis.Nanoscale Adv. 2023 May 5;5(11):2865-2872. doi: 10.1039/d3na00178d. eCollection 2023 May 30. Nanoscale Adv. 2023. PMID: 37260504 Free PMC article. Review.
-
Asymmetric alkyl-alkyl coupling between electron-deficient and unactivated alkenes to access α-chiral phosphines by Ni catalysis.Sci Adv. 2025 Jun 13;11(24):eadv6571. doi: 10.1126/sciadv.adv6571. Epub 2025 Jun 13. Sci Adv. 2025. PMID: 40512845 Free PMC article.
-
Cu(i)-catalyzed enantioselective and stereospecific borylative annulation of cyclic 1,3-dione-tethered 1,3-enynes.Chem Sci. 2025 Jul 14;16(33):14988-14994. doi: 10.1039/d5sc03007b. eCollection 2025 Aug 20. Chem Sci. 2025. PMID: 40698169 Free PMC article.
-
19F NMR-Based Chiral Analysis of Organoboron Compounds via Chiral Recognition of Fluorine-Labeled Boronates with Cobalt Complexes.JACS Au. 2024 Sep 16;4(10):3771-3776. doi: 10.1021/jacsau.4c00703. eCollection 2024 Oct 28. JACS Au. 2024. PMID: 39483235 Free PMC article.
-
Copper-catalysed oxy-alkylation of styrenes enabled by halogen-atom transfer.Chem Sci. 2025 Aug 26. doi: 10.1039/d5sc03774c. Online ahead of print. Chem Sci. 2025. PMID: 40896313 Free PMC article.
References
-
- de Meijere, A. Bräse, S. & Oestreich, M. (eds). Metal Catalyzed Cross-Coupling Reactions and More (Wiley-VCH, Weinheim, Germany, 2014).
-
- Agrawal T, Sieber JD. Recent developments in C–C bond formation using catalytic reductive coupling strategies. Synthesis. 2020;52:2623. doi: 10.1055/s-0040-1707128. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

