Copper-catalysed asymmetric reductive cross-coupling of prochiral alkenes
- PMID: 35545634
- PMCID: PMC9095606
- DOI: 10.1038/s41467-022-30286-8
Copper-catalysed asymmetric reductive cross-coupling of prochiral alkenes
Abstract
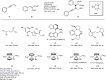
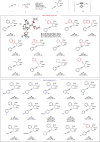
Asymmetric construction of C(sp3)-C(sp3) bond with good stereocontrol of the two connecting carbon centres retaining all carbon or hydrogen substituents is a challenging target in transition metal catalysis. Transition metal-catalysed reductive coupling of unsaturated π-substrates is considered as a potent tool to expediently develop the molecular complexity with high atom efficiency. However, such an asymmetric and intermolecular process has yet to be developed fully. Herein, we report an efficient strategy to reductively couple two prochiral conjugate alkenes using a copper-catalysed tandem protocol in the presence of diboron. Notably, this transformation incorporates a wide range of terminal and internal enynes as coupling partners and facilitates highly diastereo- and enantioselective synthesis of organoboron derivatives with multiple adjacent stereocentres in a single operation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- de Meijere, A. Bräse, S. & Oestreich, M. (eds). Metal Catalyzed Cross-Coupling Reactions and More (Wiley-VCH, Weinheim, Germany, 2014).
-
- Agrawal T, Sieber JD. Recent developments in C–C bond formation using catalytic reductive coupling strategies. Synthesis. 2020;52:2623. doi: 10.1055/s-0040-1707128. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

