Bacteroides ovatus-mediated CD27- MAIT cell activation is associated with obesity-related T2D progression
- PMID: 35545662
- PMCID: PMC9243016
- DOI: 10.1038/s41423-022-00871-4
Bacteroides ovatus-mediated CD27- MAIT cell activation is associated with obesity-related T2D progression
Abstract
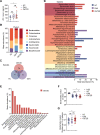
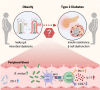
Type 2 diabetes (T2D) is highly associated with obesity. However, the factors that drive the transition from excessive weight gain to glucose metabolism disruption are still uncertain and seem to revolve around systemic immune disorder. Mucosal-associated invariant T (MAIT) cells, which are innate-like T cells that recognize bacterial metabolites, have been reported to be altered in obese people and to lead to metabolic dysfunction during obesity. By studying the immunophenotypes of blood MAIT cells from a cross-sectional cohort of obese participants with/without T2D, we found an elevation in CD27-negative (CD27-) MAIT cells producing a high level of IL-17 under T2D obese conditions, which could be positively correlated with impaired glucose metabolism in obese people. We further explored microbial translocation caused by gut barrier dysfunction in obese people as a triggering factor of MAIT cell abnormalities. Specifically, accumulation of the bacterial strain Bacteroides ovatus in the peripheral blood drove IL-17-producing CD27- MAIT cell expansion and could be associated with T2D risk in obese individuals. Overall, these results suggest that an aberrant gut microbiota-immune axis in obese people may drive or exacerbate T2D. Importantly, CD27- MAIT cell subsets and Bacteroides ovatus could represent targets for novel interventional strategies. Our findings extend current knowledge regarding the clinical relevance of body mass index (BMI)-associated variation in circulating MAIT cells to reveal the role of these cells in obesity-related T2D progression and the underlying cellular mechanisms.
Keywords: Bacteroides ovatus; MAIT cells; bacterial translocation; obesity; type 2 diabetes.
© 2022. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients.J Clin Invest. 2015 Apr;125(4):1752-62. doi: 10.1172/JCI78941. Epub 2015 Mar 9. J Clin Invest. 2015. PMID: 25751065 Free PMC article.
-
Mucosal-associated invariant T cell alterations during the development of human type 1 diabetes.Diabetologia. 2020 Nov;63(11):2396-2409. doi: 10.1007/s00125-020-05257-7. Epub 2020 Sep 3. Diabetologia. 2020. PMID: 32880687 Free PMC article.
-
Mucosal-associated invariant T cells are associated with insulin resistance in childhood obesity, and disrupt insulin signalling via IL-17.Diabetologia. 2022 Jun;65(6):1012-1017. doi: 10.1007/s00125-022-05682-w. Epub 2022 Mar 19. Diabetologia. 2022. PMID: 35305128 Free PMC article.
-
iNKT and MAIT Cell Alterations in Diabetes.Front Immunol. 2015 Jul 2;6:341. doi: 10.3389/fimmu.2015.00341. eCollection 2015. Front Immunol. 2015. PMID: 26191063 Free PMC article. Review.
-
Interleukin-17 producing mucosal associated invariant T cells - emerging players in chronic inflammatory diseases?Eur J Immunol. 2020 Aug;50(8):1098-1108. doi: 10.1002/eji.202048645. Epub 2020 Jul 15. Eur J Immunol. 2020. PMID: 32617963 Review.
Cited by
-
A microbial causal mediation analytic tool for health disparity and applications in body mass index.Res Sq [Preprint]. 2023 Jan 13:rs.3.rs-2463503. doi: 10.21203/rs.3.rs-2463503/v1. Res Sq. 2023. Update in: Microbiome. 2023 Jul 27;11(1):164. doi: 10.1186/s40168-023-01608-9. PMID: 36712075 Free PMC article. Updated. Preprint.
-
Identifying and ranking causal microbial biomarkers for colorectal cancer at different cancer subsites and stages: a Mendelian randomization study.Front Oncol. 2023 Jul 19;13:1224705. doi: 10.3389/fonc.2023.1224705. eCollection 2023. Front Oncol. 2023. PMID: 37538123 Free PMC article.
-
Classic costimulatory interactions in MAIT cell responses: from gene expression to immune regulation.Clin Exp Immunol. 2023 Jul 5;213(1):50-66. doi: 10.1093/cei/uxad061. Clin Exp Immunol. 2023. PMID: 37279566 Free PMC article.
-
MAIT cells and the microbiome.Front Immunol. 2023 Feb 23;14:1127588. doi: 10.3389/fimmu.2023.1127588. eCollection 2023. Front Immunol. 2023. PMID: 36911683 Free PMC article. Review.
-
Mucosal-associated invariant T cells correlate with myocardial ischaemia and remodelling in coronary artery disease.Clin Transl Immunology. 2025 Mar 24;14(3):e70029. doi: 10.1002/cti2.70029. eCollection 2025. Clin Transl Immunology. 2025. PMID: 40130221 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

