Genetic and chemotherapeutic influences on germline hypermutation
- PMID: 35545669
- PMCID: PMC9117138
- DOI: 10.1038/s41586-022-04712-2
Genetic and chemotherapeutic influences on germline hypermutation
Abstract
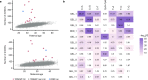
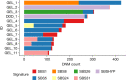
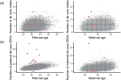
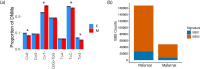
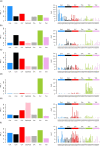
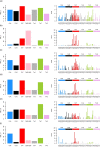
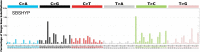
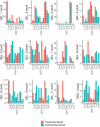
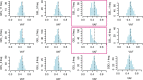
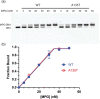
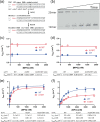
Mutations in the germline generates all evolutionary genetic variation and is a cause of genetic disease. Parental age is the primary determinant of the number of new germline mutations in an individual's genome1,2. Here we analysed the genome-wide sequences of 21,879 families with rare genetic diseases and identified 12 individuals with a hypermutated genome with between two and seven times more de novo single-nucleotide variants than expected. In most families (9 out of 12), the excess mutations came from the father. Two families had genetic drivers of germline hypermutation, with fathers carrying damaging genetic variation in DNA-repair genes. For five of the families, paternal exposure to chemotherapeutic agents before conception was probably a key driver of hypermutation. Our results suggest that the germline is well protected from mutagenic effects, hypermutation is rare, the number of excess mutations is relatively modest and most individuals with a hypermutated genome will not have a genetic disease.
© 2022. The Author(s).
Conflict of interest statement
M.H. is a co-founder of, consultant to and holds shares in Congenica, a genetics diagnostic company. L.M. and C.O. are employees of Genomics England Ltd.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

