Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes
- PMID: 35545676
- PMCID: PMC9186297
- DOI: 10.1038/s41586-022-04717-x
Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes
Abstract
Gasdermins (GSDMs) are a family of pore-forming effectors that permeabilize the cell membrane during the cell death program pyroptosis1. GSDMs are activated by proteolytic removal of autoinhibitory carboxy-terminal domains, typically by caspase regulators1-9. However, no activator is known for one member of this family, GSDMA. Here we show that the major human pathogen group A Streptococcus (GAS) secretes a protease virulence factor, SpeB, that induces GSDMA-dependent pyroptosis. SpeB cleavage of GSDMA releases an active amino-terminal fragment that can insert into membranes to form lytic pores. GSDMA is primarily expressed in the skin10, and keratinocytes infected with SpeB-expressing GAS die of GSDMA-dependent pyroptosis. Mice have three homologues of human GSDMA, and triple-knockout mice are more susceptible to invasive infection by a pandemic hypervirulent M1T1 clone of GAS. These results indicate that GSDMA is critical in the immune defence against invasive skin infections by GAS. Furthermore, they show that GSDMs can act independently of host regulators as direct sensors of exogenous proteases. As SpeB is essential for tissue invasion and survival within skin cells, these results suggest that GSDMA can act akin to a guard protein that directly detects concerning virulence activities of microorganisms that present a severe infectious threat.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures
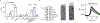


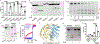


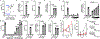
References
-
- Broz P, Pelegrín P & Shao F The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2019) - PubMed
-
- Zhou Z et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 (2020). - PubMed
-
- Ding J et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 538, 111–116 (2016) - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

