A hybrid open-top light-sheet microscope for versatile multi-scale imaging of cleared tissues
- PMID: 35545715
- PMCID: PMC9214839
- DOI: 10.1038/s41592-022-01468-5
A hybrid open-top light-sheet microscope for versatile multi-scale imaging of cleared tissues
Abstract
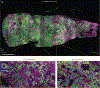
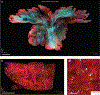
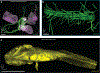
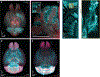
Light-sheet microscopy has emerged as the preferred means for high-throughput volumetric imaging of cleared tissues. However, there is a need for a flexible system that can address imaging applications with varied requirements in terms of resolution, sample size, tissue-clearing protocol, and transparent sample-holder material. Here, we present a 'hybrid' system that combines a unique non-orthogonal dual-objective and conventional (orthogonal) open-top light-sheet (OTLS) architecture for versatile multi-scale volumetric imaging. We demonstrate efficient screening and targeted sub-micrometer imaging of sparse axons within an intact, cleared mouse brain. The same system enables high-throughput automated imaging of multiple specimens, as spotlighted by a quantitative multi-scale analysis of brain metastases. Compared with existing academic and commercial light-sheet microscopy systems, our hybrid OTLS system provides a unique combination of versatility and performance necessary to satisfy the diverse requirements of a growing number of cleared-tissue imaging applications.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests
A.K.G., N.P.R., L.D.T., and J.T.C.L. are co-founders and shareholders of Alpenglow Biosciences. L.A.G.L. and H.L. are employees of Leica Microsystems, Inc., maker of the Aivia software.
Figures






Comment in
-
Imaging cleared tissues made easy.Nat Methods. 2022 May;19(5):527-529. doi: 10.1038/s41592-022-01424-3. Nat Methods. 2022. PMID: 35545718 No abstract available.
References
References (Main Text)
-
- Tanaka N, et al. , Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nature Biomedical Engineering, 2017. 1(10): p. 796. - PubMed
-
- Pan C, et al. , Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nat Methods, 2016. 13(10): p. 859–67. - PubMed
-
- Renier N, et al. , iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell, 2014. 159(4): p. 896–910. - PubMed
References (Online Methods)
-
- Dunsby C, Optically sectioned imaging by oblique plane microscopy. Optics Express, 2008. 16(25): p. 20306–20316. - PubMed
-
- Botcherby EJ, et al. , An optical technique for remote focusing in microscopy. Optics Communications, 2008. 281(4): p. 880–887.
-
- Millett-Sikking A, et al. High NA single-objective light-sheet. 2019; Available from: https://andrewgyork.github.io/high_na_single_objective_lightsheet/index.....
Publication types
MeSH terms
Grants and funding
- R01 CA244170/CA/NCI NIH HHS/United States
- P 31263/FWF_/Austrian Science Fund FWF/Austria
- R01 DK107436/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R00 CA240681/CA/NCI NIH HHS/United States
- K99 CA240681/CA/NCI NIH HHS/United States
- R01 GM079712/GM/NIGMS NIH HHS/United States
- R01 NS104949/NS/NINDS NIH HHS/United States
- P20 GM104318/GM/NIGMS NIH HHS/United States
- RF1 MH117820/MH/NIMH NIH HHS/United States
- R01 DK092202/DK/NIDDK NIH HHS/United States
- R01 EB031002/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

