Formate-driven H2 production by whole cells of Thermoanaerobacter kivui
- PMID: 35545791
- PMCID: PMC9097184
- DOI: 10.1186/s13068-022-02147-5
Formate-driven H2 production by whole cells of Thermoanaerobacter kivui
Abstract
Background: In times of global warming there is an urgent need to replace fossil fuel-based energy vectors by less carbon dioxide (CO2)-emitting alternatives. One attractive option is the use of molecular hydrogen (H2) since its combustion emits water (H2O) and not CO2. Therefore, H2 is regarded as a non-polluting fuel. The ways to produce H2 can be diverse, but steam reformation of conventional fossil fuel sources is still the main producer of H2 gas up to date. Biohydrogen production via microbes could be an alternative, environmentally friendly and renewable way of future H2 production, especially when the flexible and inexpensive C1 compound formate is used as substrate.
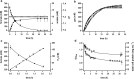
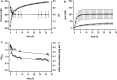
Results: In this study, the versatile compound formate was used as substrate to drive H2 production by whole cells of the thermophilic acetogenic bacterium Thermoanaerobacter kivui which harbors a highly active hydrogen-dependent CO2 reductase (HDCR) to oxidize formate to H2 and CO2 and vice versa. Under optimized reaction conditions, T. kivui cells demonstrated the highest H2 production rates (qH2 = 685 mmol g-1 h-1) which were so far reported in the literature for wild-type organisms. Additionally, high yields (Y(H2/formate)) of 0.86 mol mol-1 and a hydrogen evolution rate (HER) of 999 mmol L-1 h-1 were observed. Finally, stirred-tank bioreactor experiments demonstrated the upscaling feasibility of the applied whole cell system and indicated the importance of pH control for the reaction of formate-driven H2 production.
Conclusions: The thermophilic acetogenic bacterium T. kivui is an efficient biocatalyst for the oxidation of formate to H2 (and CO2). The existing genetic tool box of acetogenic bacteria bears further potential to optimize biohydrogen production in future and to contribute to a future sustainable formate/H2 bio-economy.
Keywords: Acetogenic bacteria; Biohydrogen; Bioreactor; Dark fermentation; HER; Hydrogen-dependent CO2 reductase (HDCR); Optimization; Scale-up; qH2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Whole-cell biocatalysis for hydrogen storage and syngas conversion to formate using a thermophilic acetogen.Biotechnol Biofuels. 2020 Feb 28;13:32. doi: 10.1186/s13068-020-1670-x. eCollection 2020. Biotechnol Biofuels. 2020. PMID: 32140177 Free PMC article.
-
Hydrogenation of CO2 at ambient pressure catalyzed by a highly active thermostable biocatalyst.Biotechnol Biofuels. 2018 Sep 1;11:237. doi: 10.1186/s13068-018-1236-3. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 30186365 Free PMC article.
-
Formate Is Required for Growth of the Thermophilic Acetogenic Bacterium Thermoanaerobacter kivui Lacking Hydrogen-Dependent Carbon Dioxide Reductase (HDCR).Front Microbiol. 2020 Jan 31;11:59. doi: 10.3389/fmicb.2020.00059. eCollection 2020. Front Microbiol. 2020. PMID: 32082286 Free PMC article.
-
New Horizons in Acetogenic Conversion of One-Carbon Substrates and Biological Hydrogen Storage.Trends Biotechnol. 2019 Dec;37(12):1344-1354. doi: 10.1016/j.tibtech.2019.05.008. Epub 2019 Jun 27. Trends Biotechnol. 2019. PMID: 31257058 Review.
-
One-carbon substrate-based biohydrogen production: microbes, mechanism, and productivity.Biotechnol Adv. 2015 Jan-Feb;33(1):165-177. doi: 10.1016/j.biotechadv.2014.11.004. Epub 2014 Nov 21. Biotechnol Adv. 2015. PMID: 25461503 Review.
Cited by
-
How FocA facilitates fermentation and respiration of formate by Escherichia coli.J Bacteriol. 2025 Feb 20;207(2):e0050224. doi: 10.1128/jb.00502-24. Epub 2025 Jan 27. J Bacteriol. 2025. PMID: 39868885 Free PMC article. Review.
-
Extremophiles in a changing world.Extremophiles. 2024 Apr 29;28(2):26. doi: 10.1007/s00792-024-01341-7. Extremophiles. 2024. PMID: 38683238 Free PMC article. Review.
-
Leveraging substrate flexibility and product selectivity of acetogens in two-stage systems for chemical production.Microb Biotechnol. 2023 Feb;16(2):218-237. doi: 10.1111/1751-7915.14172. Epub 2022 Dec 4. Microb Biotechnol. 2023. PMID: 36464980 Free PMC article. Review.
-
Utilization of formic acid by extremely thermoacidophilic archaea species.Microb Biotechnol. 2024 Sep;17(9):e70003. doi: 10.1111/1751-7915.70003. Microb Biotechnol. 2024. PMID: 39215388 Free PMC article.
-
Redirecting electron flow in Acetobacterium woodii enables growth on CO and improves growth on formate.Nat Commun. 2024 Jun 26;15(1):5424. doi: 10.1038/s41467-024-49680-5. Nat Commun. 2024. PMID: 38926344 Free PMC article.
References
-
- Dufour J, Serrano DP, Galvez JL, Moreno J, Garcia C. Life cycle assessment of processes for hydrogen production. Environmental feasibility and reduction of greenhouse gases emissions. Int J Hydrog Energy. 2009;34:1370–1376. doi: 10.1016/j.ijhydene.2008.11.053. - DOI
-
- Hay JXW, Wu TY, Juan JC, Md Jahim J. Biohydrogen production through photo fermentation or dark fermentation using waste as a substrate: overview, economics, and future prospects of hydrogen usage. Biofuels Bioprod Biorefin. 2013;7:334–352. doi: 10.1002/bbb.1403. - DOI
-
- Holladay JD, Hu J, King DL, Wang Y. An overview of hydrogen production technologies. Catal Today. 2009;139:244–260. doi: 10.1016/j.cattod.2008.08.039. - DOI
-
- Sinha P, Pandey A. An evaluative report and challenges for fermentative biohydrogen production. Int J Hydrog Energy. 2011;36:7460–7478. doi: 10.1016/j.ijhydene.2011.03.077. - DOI
-
- Das D, Veziroǧlu TN. Hydrogen production by biological processes: a survey of literature. Int J Hydrog Energy. 2001;26:13–28. doi: 10.1016/S0360-3199(00)00058-6. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
