Peroxidase-Mimicking Activity of Biogenic Gold Nanoparticles Produced from Prunus nepalensis Fruit Extract: Characterizations and Application for the Detection of Mycobacterium bovis
- PMID: 35545815
- PMCID: PMC9214696
- DOI: 10.1021/acsabm.2c00180
Peroxidase-Mimicking Activity of Biogenic Gold Nanoparticles Produced from Prunus nepalensis Fruit Extract: Characterizations and Application for the Detection of Mycobacterium bovis
Abstract
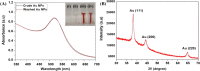
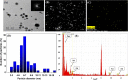
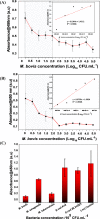
In the present study, a facile, eco-friendly, and controlled synthesis of gold nanoparticles (Au NPs) using Prunus nepalensis fruit extract is reported. The biogenically synthesized Au NPs possess ultra-active intrinsic peroxidase-like activity for the oxidation of 3,3',5,5'-tetramethylbenzidine (TMB) in the presence of H2O2. Chemical analysis of the fruit extract demonstrated the presence of various bioactive molecules such as amino acids (l-alanine and aspartic acids), organic acids (benzoic acid and citric acid), sugars (arabinose and glucose), phenolic acid, and bioflavonoids (niacin and myo-inositol), which likely attributed to the formation of stable biogenic Au NPs with excellent peroxidase-mimicking activity. In comparison with the natural horseradish peroxidase (HRP) enzyme, the biogenic Au NPs displayed a 9.64 times higher activity with regard to the reaction velocity at 6% (v/v) H2O2, presenting a higher affinity toward the TMB substrate. The Michaelis-Menten constant (KM) values for the biogenic Au NPs and HRP were found to be 6.9 × 10-2 and 7.9 × 10-2 mM, respectively, at the same concentration of 100 pM. To investigate its applicability for biosensing, a monoclonal antibody specific for Mycobacterium bovis (QUBMA-Bov) was directly conjugated to the surface of the biogenic Au NPs. The obtained results indicate that the biogenic Au NPs-QUBMA-Bov conjugates are capable of detecting M. bovis based on a colorimetric immunosensing method within a lower range of 100 to 102 cfu mL-1 with limits of detection of ∼53 and ∼71 cfu mL-1 in an artificial buffer solution and in a soft cheese spiked sample, respectively. This strategy demonstrates decent specificity in comparison with those of other bacterial and mycobacterial species. Considering these findings together, this study indicates the potential for the development of a cost-effective biosensing platform with high sensitivity and specificity for the detection of M. bovis using antibody-conjugated Au nanozymes.
Keywords: Mycobacterium bovis; biogenic nanoparticles; biosensing; green synthesis; indirect enzyme-linked immunosorbent assay (iELISA); peroxidase-mimicking.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Slocik J. M.; Naik R. R.; Stone M. O.; Wright D. W. Viral Templates for Gold Nanoparticle Synthesis. J. Mater. Chem. 2005, 15, 749–753. 10.1039/b413074j. - DOI
-
- Yang N.; Weihong L.; Hao L. Biosynthesis of Au Nanoparticles Using Agricultural Waste Mango Peel Extract and Its in Vitro Cytotoxic Effect on Two Normal Cells. Mater. Lett. 2014, 134, 67–70. 10.1016/j.matlet.2014.07.025. - DOI
-
- Raghunandan D.; Basavaraja S.; Mahesh B.; Balaji S.; Manjunath S. Y.; Venkataraman A. Biosynthesis of Stable Polyshaped Gold Nanoparticles from Microwave-Exposed Aqueous Extracellular Anti-Malignant Guava (Psidium Guajava) Leaf Extract. Nanobiotechnology 2009, 5, 34–41. 10.1007/s12030-009-9030-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

