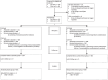
Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients
- PMID: 35546145
- PMCID: PMC9095637
- DOI: 10.1038/s41467-022-29911-3
Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients
Erratum in
-
Author Correction: Prospective individual patient data meta-analysis of two randomized trials on convalescent plasma for COVID-19 outpatients.Nat Commun. 2024 May 22;15(1):4352. doi: 10.1038/s41467-024-48645-y. Nat Commun. 2024. PMID: 38778041 Free PMC article. No abstract available.
Abstract
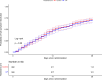
Data on convalescent plasma (CP) treatment in COVID-19 outpatients are scarce. We aimed to assess whether CP administered during the first week of symptoms reduced the disease progression or risk of hospitalization of outpatients. Two multicenter, double-blind randomized trials (NCT04621123, NCT04589949) were merged with data pooling starting when <20% of recruitment target was achieved. A Bayesian-adaptive individual patient data meta-analysis was implemented. Outpatients aged ≥50 years and symptomatic for ≤7days were included. The intervention consisted of 200-300mL of CP with a predefined minimum level of antibodies. Primary endpoints were a 5-point disease severity scale and a composite of hospitalization or death by 28 days. Amongst the 797 patients included, 390 received CP and 392 placebo; they had a median age of 58 years, 1 comorbidity, 5 days symptoms and 93% had negative IgG antibody-test. Seventy-four patients were hospitalized, 6 required mechanical ventilation and 3 died. The odds ratio (OR) of CP for improved disease severity scale was 0.936 (credible interval (CI) 0.667-1.311); OR for hospitalization or death was 0.919 (CI 0.592-1.416). CP effect on hospital admission or death was largest in patients with ≤5 days of symptoms (OR 0.658, 95%CI 0.394-1.085). CP did not decrease the time to full symptom resolution.
Trial registration: Clinicaltrials.gov NCT04621123 and NCT04589949.
Registration: NCT04621123 and NCT04589949 on https://www.
Clinicaltrials: gov.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures