Neurophysiological effects of human-derived pathological tau conformers in the APPKM670/671NL.PS1/L166P amyloid mouse model of Alzheimer's disease
- PMID: 35546164
- PMCID: PMC9094605
- DOI: 10.1038/s41598-022-11582-1
Neurophysiological effects of human-derived pathological tau conformers in the APPKM670/671NL.PS1/L166P amyloid mouse model of Alzheimer's disease
Abstract
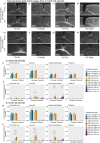
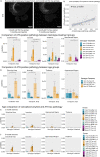
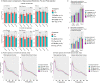
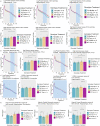
Alzheimer's Disease (AD) is a neurodegenerative disease characterized by two main pathological hallmarks: amyloid plaques and intracellular tau neurofibrillary tangles. However, a majority of studies focus on the individual pathologies and seldom on the interaction between the two pathologies. Herein, we present the longitudinal neuropathological and neurophysiological effects of a combined amyloid-tau model by hippocampal seeding of human-derived tau pathology in the APP.PS1/L166P amyloid animal model. We statistically assessed both neurophysiological and pathological changes using linear mixed modelling to determine if factors such as the age at which animals were seeded, genotype, seeding or buffer, brain region where pathology was quantified, and time-post injection differentially affect these outcomes. We report that AT8-positive tau pathology progressively develops and is facilitated by the amount of amyloid pathology present at the time of injection. The amount of AT8-positive tau pathology was influenced by the interaction of age at which the animal was injected, genotype, and time after injection. Baseline pathology-related power spectra and Higuchi Fractal Dimension (HFD) score alterations were noted in APP.PS1/L166P before any manipulations were performed, indicating a baseline difference associated with genotype. We also report immediate localized hippocampal dysfunction in the electroencephalography (EEG) power spectra associated with tau seeding which returned to comparable levels at 1 month-post-injection. Longitudinal effects of seeding indicated that tau-seeded wild-type mice showed an increase in gamma power earlier than buffer control comparisons which was influenced by the age at which the animal was injected. A reduction of hippocampal broadband power spectra was noted in tau-seeded wild-type mice, but absent in APP.PS1 animals. HFD scores appeared to detect subtle effects associated with tau seeding in APP.PS1 animals, which was differentially influenced by genotype. Notably, while tau histopathological changes were present, a lack of overt longitudinal electrophysiological alterations was noted, particularly in APP.PS1 animals that feature both pathologies after seeding, reiterating and underscoring the difficulty and complexity associated with elucidating physiologically relevant and translatable biomarkers of Alzheimer's Disease at the early stages of the disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they are working for Janssen Pharmaceutica NV. The authors declare no other commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

