The early-life stress induced by oxytocin inhibition in p53 knockout mouse dams increases adulthood tumorigenesis in first and second generations
- PMID: 35546267
- PMCID: PMC9875680
- DOI: 10.1002/cnr2.1625
The early-life stress induced by oxytocin inhibition in p53 knockout mouse dams increases adulthood tumorigenesis in first and second generations
Abstract
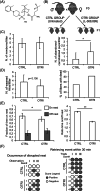
Background: Early-life stress due to poor parental care has been suggested to increase cancer risk, though, so far, no experimental evidence established a link between defective parental behavior and spontaneous tumorigenesis in progeny. Essential maternal behavior is regulated, in particular, by the oxytocin (OT) hormonal circuit, which in turn responds to stimuli from the offspring and impinges on the central nervous systems.
Methods: By providing L-368,899 OT receptor (OTR) inhibitor to lactating mothers, we set up a model of defective maternal care in p53 knockout mice.
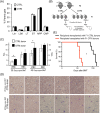
Results: The progeny of these dams showed, later in life, higher cortisol levels, shortened life span and increased tumorigenic potential of bone marrow cells (BMC). Notably, these phenotypes were transmitted to the following generation.
Conclusions: Therefore, the inhibition of OT function in mothers is a novel paradigm of early-life stress that is inherited across generations and increases cancer risk in tumor-prone mice.
Keywords: cancer risk factor; early-life stress; oxytocin; p53 KO mice; parental care.
© 2022 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

