The Current Status of Clinical Trials on Biologics for Cartilage Repair and Osteoarthritis Treatment: An Analysis of ClinicalTrials.gov Data
- PMID: 35546280
- PMCID: PMC9152205
- DOI: 10.1177/19476035221093065
The Current Status of Clinical Trials on Biologics for Cartilage Repair and Osteoarthritis Treatment: An Analysis of ClinicalTrials.gov Data
Abstract
Objective: Biologics are increasingly used for cartilage repair and osteoarthritis (OA) treatment. This study aimed to provide an overview of the clinical trials conducted on this subject.
Design: Two-word combinations of two sets of key words "cartilage"; "joint"; "osteoarthritis" and "biologics"; "stem cells"; "cell implantation" were used to search the database of ClinicalTrials.gov and supplemented with searches of PubMed and EMbase. The registered trials were analyzed for clinical conditions, completion status, phases, and investigated biologics. Recently completed trials with posted/published results were summarized.
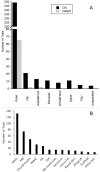
Results: From 2000 to 2022, a total of 365 clinical trials were registered at ClinicalTrials.gov to use biologics for cartilage repair and OA treatment. Since 2006, the number of registered trials accelerated at an annual rate of 16.4%. Of the 265 trials designated with a phase, 72% were early Phase 1, Phase 1, and Phase 2. Chondrocytes and platelet-rich plasma (PRP) were studied in nearly equal number of early- and late-stage trials. Mesenchymal stem/stromal cells (MSCs) were the most commonly investigated biologics (38%) and mostly derived from bone marrow and adipose tissue (70%). In last 5 years, 32 of the 72 completed trials posted/published results, among which seven Phase 3 trials investigated chondrocytes, PRP, bone marrow aspirate concentrate, hyaluronic acid, collagen membrane, and albumin.
Conclusions: There was a rapid increase in the number of registered clinical trials in recent years, using a variety of biologics for cartilage repair and OA treatment. Majority of the biologics still require late-stage trials to validate their clinical effectiveness.
Keywords: biologics; cartilage; clinical trial; osteoarthritis; registry.
Conflict of interest statement
Figures

References
-
- Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79. - PubMed
-
- Southworth TM, Naveen NB, Nwachukwu BU, Cole BJ, Frank RM. Orthobiologics for focal articular cartilage defects. Clin Sports Med. 2019;38(1):109-22. - PubMed
-
- Weber AE, Bolia IK, Trasolini NA. Biological strategies for osteoarthritis: from early diagnosis to treatment. Int Orthop. 2021;45(2):335-44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

