Interplay between hypertriglyceridemia and acute promyelocytic leukemia mediated by the cooperation of peroxisome proliferator-activated receptor-α with the PML/RAR α fusion protein on super-enhancers
- PMID: 35546300
- PMCID: PMC9614538
- DOI: 10.3324/haematol.2021.280147
Interplay between hypertriglyceridemia and acute promyelocytic leukemia mediated by the cooperation of peroxisome proliferator-activated receptor-α with the PML/RAR α fusion protein on super-enhancers
Abstract
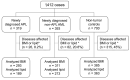
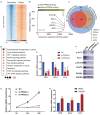
Patients with newly diagnosed acute promyelocytic leukemia (APL) are often obese or overweight, accompanied by metabolic disorders, such as dyslipidemia. However, the link between dyslipidemia and leukemia is obscure. Here, we conducted a retrospective study containing 1,412 cases (319 newly diagnosed APL patients, 393 newly diagnosed non-APL acute myeloid leukemia patients, and 700 non-tumor controls) and found that APL patients had higher triglyceride levels than non- APL and control groups. Using clinical data, we revealed that hypertriglyceridemia served as a risk factor for early death in APL patients, and there was a positive correlation between triglyceride levels and leukocyte counts. RNA sequencing analysis of APL patients having high or normal triglyceride levels highlighted the contribution of peroxisome proliferatoractivated receptor-α (PPARα), a crucial regulator of cell metabolism and a transcription factor involved in cancer development. The genome-wide chromatin occupancy of PPARα revealed that PPARα co-existed with PML/RARα within the super-enhancer regions to promote cell proliferation. PPARα knockdown affected the expression of target genes responsible for APL proliferation, including FLT3, and functionally inhibited the proliferation of APL cells. Moreover, in vivo results in mice having high fat diet-induced high triglyceride levels supported the connection between high triglyceride levels and the leukemic burden, as well as the involvement of PPARα-mediated-FLT3 activation in the proliferation of APL cells. Our findings shed light on the association between APL proliferation and high triglyceride levels and provide a genetic link to PPARα-mediated hyperlipidemia in APL.
Figures




References
-
- Estey E, Thall P, Kantarjian H, Pierce S, Kornblau S, Keating M. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11(10):1661-1664. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

