Ferric Citrate Uptake Is a Virulence Factor in Uropathogenic Escherichia coli
- PMID: 35546538
- PMCID: PMC9239202
- DOI: 10.1128/mbio.01035-22
Ferric Citrate Uptake Is a Virulence Factor in Uropathogenic Escherichia coli
Abstract
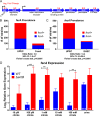
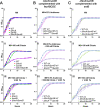
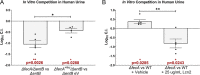
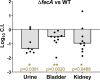
More than half of women will experience a urinary tract infection (UTI), with uropathogenic Escherichia coli (UPEC) causing ~80% of uncomplicated cases. Iron acquisition systems are essential for uropathogenesis, and UPEC strains encode highly diverse iron acquisition systems, underlining their importance. However, a recent UPEC clinical isolate, HM7, lacks this diversity and instead encodes the synthesis pathway for a sole siderophore, enterobactin. To determine if HM7 possesses unidentified iron acquisition systems, we performed RNA sequencing under iron-limiting conditions and demonstrated that the ferric citrate uptake system (fecABCDE and fecIR) was highly upregulated. Importantly, there are high levels of citrate within urine, some of which is bound to iron, and the fec system is enriched in UPEC isolates compared to fecal strains. Therefore, we hypothesized that HM7 and other similar strains use the fec system to acquire iron in the host. Deletion of both enterobactin biosynthesis and ferric citrate uptake (ΔfecA/ΔentB) abrogates use of ferric citrate as an iron source, and fecA provides an advantage in human urine in the absence of enterobactin. However, in a UTI mouse model, fecA is a fitness factor independent of enterobactin production, likely due to the action of host lipocalin-2 chelating ferrienterobactin. These findings indicate that ferric citrate uptake is used as an iron source when siderophore efficacy is limited, such as in the host during UTI. Defining these novel compensatory mechanisms and understanding the nutritional hierarchy of preferred iron sources within the urinary tract are important in the search for new approaches to combat UTI. IMPORTANCE UPEC, the primary causative agent of uncomplicated UTI, is responsible for five billion dollars in health care costs in the United States each year. Rates of antibiotic resistance are on the rise; therefore, it is vital to understand the mechanisms of UPEC pathogenesis to uncover potential targets for novel therapeutics. Iron acquisition systems used to obtain iron from sequestered host sources are essential for UPEC survival during UTI and have been used as vaccine targets to prevent infection. This study reveals the ferric citrate uptake system is another important iron acquisition system that is highly enriched in UPEC strains. Ferric citrate uptake has not previously been associated with UPEC isolates, underlining the importance of the continued study of these strains to fully understand their mechanisms of pathogenesis.
Keywords: Escherichia coli; iron acquisition; iron transport; pathogenesis; siderophores; urinary tract infection; virulence factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- O'Hanley P. 1996. Prospects for urinary tract infection vaccines, p 405–425. In Mobley HLT, Warren JW (ed), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, DC.
-
- Litwin MS, Saigal CS. 2007. Urinary Tract Infections. In Urologic Diseases in America. NIH Publication No. 12-7865, GPO, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

