Diagnostic accuracy of keystroke dynamics as digital biomarkers for fine motor decline in neuropsychiatric disorders: a systematic review and meta-analysis
- PMID: 35546606
- PMCID: PMC9095860
- DOI: 10.1038/s41598-022-11865-7
Diagnostic accuracy of keystroke dynamics as digital biomarkers for fine motor decline in neuropsychiatric disorders: a systematic review and meta-analysis
Abstract
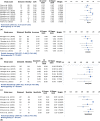
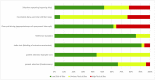
The unmet timely diagnosis requirements, that take place years after substantial neural loss and neuroperturbations in neuropsychiatric disorders, affirm the dire need for biomarkers with proven efficacy. In Parkinson's disease (PD), Mild Cognitive impairment (MCI), Alzheimers disease (AD) and psychiatric disorders, it is difficult to detect early symptoms given their mild nature. We hypothesize that employing fine motor patterns, derived from natural interactions with keyboards, also knwon as keystroke dynamics, could translate classic finger dexterity tests from clinics to populations in-the-wild for timely diagnosis, yet, further evidence is required to prove this efficiency. We have searched PubMED, Medline, IEEEXplore, EBSCO and Web of Science for eligible diagnostic accuracy studies employing keystroke dynamics as an index test for the detection of neuropsychiatric disorders as the main target condition. We evaluated the diagnostic performance of keystroke dynamics across 41 studies published between 2014 and March 2022, comprising 3791 PD patients, 254 MCI patients, and 374 psychiatric disease patients. Of these, 25 studies were included in univariate random-effect meta-analysis models for diagnostic performance assessment. Pooled sensitivity and specificity are 0.86 (95% Confidence Interval (CI) 0.82-0.90, I2 = 79.49%) and 0.83 (CI 0.79-0.87, I2 = 83.45%) for PD, 0.83 (95% CI 0.65-1.00, I2 = 79.10%) and 0.87 (95% CI 0.80-0.93, I2 = 0%) for psychomotor impairment, and 0.85 (95% CI 0.74-0.96, I2 = 50.39%) and 0.82 (95% CI 0.70-0.94, I2 = 87.73%) for MCI and early AD, respectively. Our subgroup analyses conveyed the diagnosis efficiency of keystroke dynamics for naturalistic self-reported data, and the promising performance of multimodal analysis of naturalistic behavioral data and deep learning methods in detecting disease-induced phenotypes. The meta-regression models showed the increase in diagnostic accuracy and fine motor impairment severity index with age and disease duration for PD and MCI. The risk of bias, based on the QUADAS-2 tool, is deemed low to moderate and overall, we rated the quality of evidence to be moderate. We conveyed the feasibility of keystroke dynamics as digital biomarkers for fine motor decline in naturalistic environments. Future work to evaluate their performance for longitudinal disease monitoring and therapeutic implications is yet to be performed. We eventually propose a partnership strategy based on a "co-creation" approach that stems from mechanistic explanations of patients' characteristics derived from data obtained in-clinics and under ecologically valid settings. The protocol of this systematic review and meta-analysis is registered in PROSPERO; identifier CRD42021278707. The presented work is supported by the KU-KAIST joint research center.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

