Recent advances in syntheses, properties and applications of TiO2 nanostructures
- PMID: 35546837
- PMCID: PMC9085470
- DOI: 10.1039/c8ra06517a
Recent advances in syntheses, properties and applications of TiO2 nanostructures
Abstract
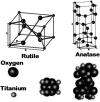
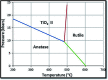
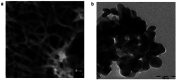
TiO2 is a compound of great importance due to its remarkable catalytic and distinctive semiconducting properties. It is also a chemically stable, non-toxic and biocompatible material. Nano TiO2 is strong oxidizing agent with a large surface area and, hence, high photo-catalytic activities. With low production cost and a high dielectric constant, it is an inexpensive material. It can be prepared by diverse procedures such as solution and gas phase procedures. Nowadays, TiO2 is being used frequently for photo degradation of organic molecules and water splitting for hydrogen generation. Most important applications include purification, disinfection of waste water, self-cleaning coatings for buildings in urban areas and the production of the green currency of energy (hydrogen) by splitting water. The review describes the advances in the syntheses, properties and applications of TiO2 nano structures. Besides, efforts are also made to discuss the working mechanism and future challenges and perspectives.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict of interest.
Figures
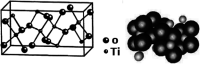












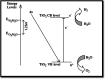

References
-
- Kaida T. Kobayashi K. Adachi M. Suzuki F. J. Cosmet. Sci. 2004;55:219–220. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

