Preparation of Ru(ii)@oligonucleotide nanosized polymers as potential tumor-imaging luminescent probes
- PMID: 35546841
- PMCID: PMC9085494
- DOI: 10.1039/c8ra05454a
Preparation of Ru(ii)@oligonucleotide nanosized polymers as potential tumor-imaging luminescent probes
Abstract
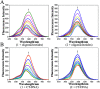
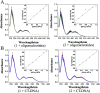
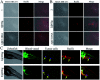
The development of Ru(ii) complexes as luminescent probes has attracted increasing attention in recent decades. In this study, the nanosized polymers of two Ru(ii) complexes [Ru(phen)2(dppz)](ClO4)2 (1, phen = 1,10-phenanthrolin; dppz = dipyrido[3,2-a:2',3'-c]phenazine) and [Ru(phen)2(Br-dppz)](ClO4)2 (2, Br-dppz = 11-bromodipyrido[3,2-a:2',3'-c]phenazine) with oligonucleotides were prepared and investigated as potential tumor-imaging probes. The formation of the nanosized polymers, which had an average width of 125-438 nm and an average height of 3-6 nm, for 1 and 2@oligonucleotides were observed through atomic force microscopy. The emission spectra indicated that the luminescence of 1 and 2 markedly increased after binding to oligonucleotides and double-strand DNA (calf thymus DNA), respectively. Moreover, further studies indicated that 1@oligonucleotides and 2@oligonucleotides can easily enter into tumor cells and selectively highlight the tumor area in the zebrafish bear xenograft tumor (MDA-MB-231). In summary, this study demonstrated that 1@oligonucleotides and 2@oligonucleotides could be developed as potential tumor-imaging luminescent probes for clinical diagnosis and therapy.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures




References
-
- Vo-Dinh T. Wang J. Lakowicz J. R. Vernier P. T. Sun Y. Gryczynski Z. K. Gundersen M. A. Marcu L. Proc. SPIE. 2005;5703:127. doi: 10.1117/12.591385. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

