What Is the Minimum Clinically Important Change in Negative Symptoms of Schizophrenia? PANSS Based Post-hoc Analyses of a Phase III Clinical Trial
- PMID: 35546918
- PMCID: PMC9083222
- DOI: 10.3389/fpsyt.2022.816339
What Is the Minimum Clinically Important Change in Negative Symptoms of Schizophrenia? PANSS Based Post-hoc Analyses of a Phase III Clinical Trial
Abstract
Introduction: Minimum clinically important difference (MCID) is a measure that defines the minimum amount of change in an objective score of a clinical test that must be reached for that change to be clinically noticeable. We aimed to find the MCID for patients with predominantly negative symptoms of schizophrenia at its earliest occurrence.
Methods: Data of a 26-week long, double-blind study with 454 patients [Positive and Negative Symptom Scale Negative Factor Score (PANSS-FSNS) ≥24, Positive and Negative Symptom Scale Positive Factor Score (PANSS-FSPS) ≤ 19] treated with cariprazine 4.5 mg/d or risperidone 4 mg/d were analyzed. The Clinical Global Impression-Improvement scale was used to quantify minimum improvement (CGI-I = 3) and no clinical change (CGI-I = 4) on the PANSS-FSNS, and the MCID was estimated with the following methods: as the mean PANSS-FSNS changes corresponding to the first instance of minimal improvement across all visits (MCID1); as the difference between the PANSS-FSNS change associated with the first instance and the PANSS-FSNS changes associated with the last recorded clinically unchanged status across all visits (MCID2); with the effect size approach (MCID3); as the Youden Index based cut-off value between no clinical change and minimal improvement (MCID4); as the relative likelihood of minimal improvement (MCID5).
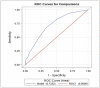
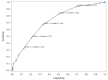
Results: The MCID1 and MCID2 resulted in, respectively, a 3.8-point (18.5%) and a 1.5-point (7.3%) decrease from baseline severity on the PANSS-FSNS. Greater values were required for the MCID at later evaluation times. The cut-off between minimum improvement and no clinical change defined by the Youden Index was a-3-point (15%) change in the PANSS-FSNS. The effect size approach indicated the 1.5-point difference between minimally improved and unchanged patients to be a medium effect (ES = 0.6).
Conclusion: Applying different methods led to different results, ranging between 7.3 and 18.5% improvement from the baseline for the MCID at its earliest occurrence in patients with predominantly negative symptoms of schizophrenia.
Keywords: MCID; cariprazine; clinical trial; minimum clinically important difference; negative symptoms; schizophrenia.
Copyright © 2022 Czobor, Sebe, Acsai, Barabássy, Laszlovszky, Németh, Furukawa and Leucht.
Conflict of interest statement
PC, BS, KA, ÁB, IL, and GN reports personal fees from Gedeon Richter Plc., outside the submitted work. TAF reports grants and personal fees from Mitsubishi-Tanabe and from Shionogi, personal fees from MSD and from SONY, outside the submitted work. In addition, TAF has a patent 2020-548587 concerning smartphone CBT apps pending, and intellectual properties for Kokoro-app licensed to Mitsubishi-Tanabe. SL reports honoraria as a consultant/advisor and/or for lectures from Angelini, Böhringer Ingelheim, Geodon Richter, Janssen, Johnson & Johnson, Lundbeck, LTS Lohmann, MSD, Otsuka, Recordati, SanofiAventis, Sandoz, Sunovion, TEVA, Eisai, Rovi, and Medichem. GN and IL have issued patents for cariprazine. This study was sponsored by Gedeon Richter Plc. Gedeon Richter was involved in the study design, collection (via contracted clinical investigator sites), analysis, and interpretation of data and decided to submit it for publication.
Figures

Similar articles
-
Linking PANSS negative symptom scores with the Clinical Global Impressions Scale: understanding negative symptom scores in schizophrenia.Neuropsychopharmacology. 2019 Aug;44(9):1589-1596. doi: 10.1038/s41386-019-0363-2. Epub 2019 Mar 5. Neuropsychopharmacology. 2019. PMID: 30836381 Free PMC article. Clinical Trial.
-
Efficacy of cariprazine on negative symptoms in patients with acute schizophrenia: A post hoc analysis of pooled data.Schizophr Res. 2019 Feb;204:282-288. doi: 10.1016/j.schres.2018.08.020. Epub 2018 Aug 29. Schizophr Res. 2019. PMID: 30172595 Clinical Trial.
-
The efficacy of cariprazine in negative symptoms of schizophrenia: Post hoc analyses of PANSS individual items and PANSS-derived factors.Eur Psychiatry. 2019 May;58:1-9. doi: 10.1016/j.eurpsy.2019.01.015. Epub 2019 Feb 7. Eur Psychiatry. 2019. PMID: 30738380 Clinical Trial.
-
Cariprazine to Treat Schizophrenia and Bipolar Disorder in Adults.Psychopharmacol Bull. 2020 Sep 14;50(4):83-117. Psychopharmacol Bull. 2020. PMID: 33012874 Free PMC article. Review.
-
Paliperidone extended release: a review of its use in the management of schizophrenia.Drugs. 2010 Jul 9;70(10):1295-317. doi: 10.2165/11204840-000000000-00000. Drugs. 2010. PMID: 20568835 Review.
Cited by
-
Crossroads of methodological choices in research synthesis: insights from two network meta-analyses on preventing relapse in schizophrenia.BMJ Ment Health. 2023 Feb;26(1):e300677. doi: 10.1136/bmjment-2023-300677. BMJ Ment Health. 2023. PMID: 37197798 Free PMC article.
-
The relationship between mindfulness and empathy with the oxytocinergic system in persons with schizophrenia spectrum disorders - A proof-of-concept randomized controlled trial (OXYGEN).Int J Clin Health Psychol. 2024 Jul-Sep;24(3):100503. doi: 10.1016/j.ijchp.2024.100503. Epub 2024 Sep 10. Int J Clin Health Psychol. 2024. PMID: 39308779 Free PMC article.
-
The minimal important difference in obsessive-compulsive disorder: An analysis of double-blind SSRI trials in adults.Eur Psychiatry. 2024 Sep 20;67(1):e53. doi: 10.1192/j.eurpsy.2024.1768. Eur Psychiatry. 2024. PMID: 39301594 Free PMC article.
-
Aripiprazole Lauroxil Every 2 Months or Paliperidone Palmitate Monthly for Acute Schizophrenia: A Post Hoc Analysis of PANSS Five-Factor Scores in the ALPINE Trial.Neuropsychiatr Dis Treat. 2025 May 14;21:1047-1055. doi: 10.2147/NDT.S510471. eCollection 2025. Neuropsychiatr Dis Treat. 2025. PMID: 40390796 Free PMC article.
-
A Case Report of Treatment With Cariprazine in a Recurrent Psychosis Presumably Induced by Methamphetamine.Cureus. 2023 Oct 16;15(10):e47135. doi: 10.7759/cureus.47135. eCollection 2023 Oct. Cureus. 2023. PMID: 38021522 Free PMC article.
References
LinkOut - more resources
Full Text Sources

