Chicken Feather Waste Valorization Into Nutritive Protein Hydrolysate: Role of Novel Thermostable Keratinase From Bacillus pacificus RSA27
- PMID: 35547122
- PMCID: PMC9083118
- DOI: 10.3389/fmicb.2022.882902
Chicken Feather Waste Valorization Into Nutritive Protein Hydrolysate: Role of Novel Thermostable Keratinase From Bacillus pacificus RSA27
Abstract
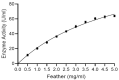
Microbial keratinases exhibit a momentous role in converting keratin biowastes into exceedingly valuable protein supplements. This study reports a novel, highly stable keratinase from Bacillus pacificus RSA27 for the production of pure peptides rich in essential amino acids from chicken feathers. Purified keratinase showed a specific activity of 38.73 U/mg, 2.58-fold purification, and molecular weight of 36 kDa. Kinetic studies using a chicken feather as substrate report K m and V max values of 5.69 mg/ml and 142.40 μg/ml/min, respectively, suggesting significant enzyme-substrate affinity/biocatalysis. Identification and in silico structural-functional analysis of keratinase discovered the presence of distinct amino acid residues and their positions. Besides, keratinase possesses a high-affinity calcium-binding site (Asp128, Leu162, Asn164, Ile166, and Val168) and a catalytic triad of Asp119, His151, and Ser308, known attributes of serine protease (subtilisin family). Furthermore, a scale-up to 5 L fermenter revealed complete feather hydrolysis (94.5%) within 24 h with high activity (789 U/ml) and total amino acid of 153.97 μmol/ml. Finally, cytotoxicity evaluation of protein hydrolysate resulted in negligible cytotoxic effects (1.02%) on the mammalian hepatoblastoma cell line, signifying its potential biotechnological applications.
Keywords: amino acid production; characterization; keratinolytic protease; purification; scale-up; structural modeling.
Copyright © 2022 Sharma, Timorshina, Osmolovskiy, Misri and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Valorization of chicken feather waste into bioactive keratin hydrolysate by a newly purified keratinase from Bacillus sp. RCM-SSR-102.J Environ Manage. 2020 Nov 1;273:111195. doi: 10.1016/j.jenvman.2020.111195. Epub 2020 Aug 6. J Environ Manage. 2020. PMID: 32771848
-
Novel Feather Degrading Keratinases from Bacillus cereus Group: Biochemical, Genetic and Bioinformatics Analysis.Microorganisms. 2022 Jan 1;10(1):93. doi: 10.3390/microorganisms10010093. Microorganisms. 2022. PMID: 35056542 Free PMC article.
-
Efficient valorization of feather waste by Bacillus cereus IIPK35 for concomitant production of antioxidant keratin hydrolysate and milk-clotting metallo-serine keratinase.J Environ Manage. 2022 Dec 15;324:116380. doi: 10.1016/j.jenvman.2022.116380. Epub 2022 Oct 5. J Environ Manage. 2022. PMID: 36208515
-
Current Understanding of Feather Keratin and Keratinase and Their Applications in Biotechnology.Biochem Res Int. 2025 Apr 22;2025:6619273. doi: 10.1155/bri/6619273. eCollection 2025. Biochem Res Int. 2025. PMID: 40308531 Free PMC article. Review.
-
Structure, Application, and Biochemistry of Microbial Keratinases.Front Microbiol. 2021 Jun 23;12:674345. doi: 10.3389/fmicb.2021.674345. eCollection 2021. Front Microbiol. 2021. PMID: 34248885 Free PMC article. Review.
Cited by
-
Keratinous bioresources: their generation, microbial degradation, and value enhancement for biotechnological applications.World J Microbiol Biotechnol. 2025 Mar 29;41(4):118. doi: 10.1007/s11274-025-04336-4. World J Microbiol Biotechnol. 2025. PMID: 40155538 Review.
-
Screening and genomic evaluation of keratinolytic protease producing Chryseobacterium sp. from tannery waste and its potential application in dehairing of goat skin.J Genet Eng Biotechnol. 2025 Mar;23(1):100458. doi: 10.1016/j.jgeb.2025.100458. Epub 2025 Jan 19. J Genet Eng Biotechnol. 2025. PMID: 40074432 Free PMC article.
-
Keratinolytic Properties of Aspergillus clavatus Promising for Biodegradation.Int J Environ Res Public Health. 2022 Oct 26;19(21):13939. doi: 10.3390/ijerph192113939. Int J Environ Res Public Health. 2022. PMID: 36360819 Free PMC article.
-
High keratinase and other types of hydrolase activity of the new strain of Bacillus paralicheniformis.PLoS One. 2024 Oct 25;19(10):e0312679. doi: 10.1371/journal.pone.0312679. eCollection 2024. PLoS One. 2024. PMID: 39453952 Free PMC article.
-
Bio-molecular analyses enable new insights into the taphonomy of feathers.PNAS Nexus. 2024 Aug 21;3(9):pgae341. doi: 10.1093/pnasnexus/pgae341. eCollection 2024 Sep. PNAS Nexus. 2024. PMID: 39228813 Free PMC article.
References
-
- Brandelli A.. (2008). Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioproc. Tech. 1, 105–116. 10.1007/s11947-007-0025-y - DOI
-
- Calin M., Constantinescu A. D., Alexandrescu E., Raut I., Badea D. M., Arsene M. L., et al. . (2017). Degradation of keratin substrates by keratinolytic fungi. Electron. J. Biotechn. 28, 101–112. 10.1016/j.ejbt.2017.05.007 - DOI
LinkOut - more resources
Full Text Sources

