Regenerative Effects of Locally or Intra-Arterially Administered BMSCs on the Thin Endometrium
- PMID: 35547156
- PMCID: PMC9081369
- DOI: 10.3389/fbioe.2022.735465
Regenerative Effects of Locally or Intra-Arterially Administered BMSCs on the Thin Endometrium
Abstract
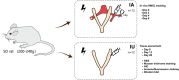
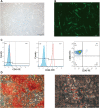
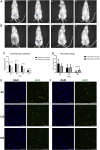
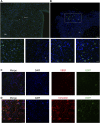
Stem cell-based therapy plays a pivotal role in the regeneration of damaged endometrium. Previous studies have demonstrated the therapeutic potential of bone marrow mesenchymal stem cells (BMSCs) through diverse administration ways. However, the homing, survival, and differentiation potential of these differently administered BMSCs are poorly defined, and the best route of administration is not well-defined. Herein, we aim to compare the engraftment, retaining time, and therapeutic efficiency of differently administered BMSCs. To achieve this, GFP/Luc-labeled BMSCs administered in two modes were assessed in a thin endometrium rat model: either into the damaged horns directly or through the ipsilateral iliac artery. The retaining time and hemi-quantitative distribution were evaluated by in vivo bioluminescence imaging and immunohistological analysis. Locally administered BMSCs were strongly detected in the abdomen at the first 4 days post treatment but underwent a rapid decrease in luminescent signal afterward and were rarely found 28 days after treatment. In contrast, the retaining time of BMSCs injected through the iliac artery was longer, reflected by more GFP-positive cells detected in the uterine section 28 days post treatment. Differentiation toward endometrial stromal cells was observed. Both routes of administration contributed to the restoration of the damaged endometrium, showing a comparable increase in the endometrial thickness and a decrease in fibrosis. However, more importantly, higher expression of LIF and VEGF, better recruitment, and longer retainment were found in the intra-arterial administration, contributing to the establishment of the optimal administration mode in clinical practice.
Keywords: angiogenesis; fibrosis; regenerative; stem cells; thin endometrium.
Copyright © 2022 Guo, Chang, Li, Zhou, Huang, Yang, Liu and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Bone Marrow Mesenchymal Stem Cells (BMSCs) Restore Functional Endometrium in the Rat Model for Severe Asherman Syndrome.Reprod Sci. 2019 Mar;26(3):436-444. doi: 10.1177/1933719118799201. Epub 2018 Nov 20. Reprod Sci. 2019. PMID: 30458678
-
Effects of bone marrow mesenchymal stem cells on repair and receptivity of damaged endometrium in rats.J Obstet Gynaecol Res. 2021 Sep;47(9):3223-3231. doi: 10.1111/jog.14888. Epub 2021 Jun 28. J Obstet Gynaecol Res. 2021. PMID: 34184363
-
Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium.Reprod Sci. 2015 Feb;22(2):181-8. doi: 10.1177/1933719114537715. Epub 2014 Jun 19. Reprod Sci. 2015. PMID: 24947483 Free PMC article.
-
Endometrial stem/progenitor cells: the first 10 years.Hum Reprod Update. 2016 Mar-Apr;22(2):137-63. doi: 10.1093/humupd/dmv051. Epub 2015 Nov 9. Hum Reprod Update. 2016. PMID: 26552890 Free PMC article. Review.
-
The Therapeutic Potential of Bone Marrow Mesenchymal Stem Cells for Articular Cartilage Regeneration in Osteoarthritis.Curr Stem Cell Res Ther. 2021;16(7):840-847. doi: 10.2174/1574888X16666210127130044. Curr Stem Cell Res Ther. 2021. PMID: 33504316 Review.
Cited by
-
β-Lactoglobulin affects the oxidative status and viability of equine endometrial progenitor cells via lncRNA-mRNA-miRNA regulatory associations.J Cell Mol Med. 2023 Apr;27(7):927-938. doi: 10.1111/jcmm.17694. Epub 2023 Mar 1. J Cell Mol Med. 2023. PMID: 36860157 Free PMC article.
-
Impaired receptivity of thin endometrium: therapeutic potential of mesenchymal stem cells.Front Endocrinol (Lausanne). 2024 Jan 25;14:1268990. doi: 10.3389/fendo.2023.1268990. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38344687 Free PMC article. Review.
-
Network Pharmacology and Molecular Docking Approach to Reveal the Immunotherapeutic Mechanism of Cuscutae Semen in Treating Thin Endometrium.J Immunol Res. 2022 Sep 22;2022:4333128. doi: 10.1155/2022/4333128. eCollection 2022. J Immunol Res. 2022. PMID: 36249421 Free PMC article.
References
LinkOut - more resources
Full Text Sources

