Efficient Synthesis of (R)-(+)-Perillyl Alcohol From (R)-(+)-Limonene Using Engineered Escherichia coli Whole Cell Biocatalyst
- PMID: 35547170
- PMCID: PMC9084310
- DOI: 10.3389/fbioe.2022.900800
Efficient Synthesis of (R)-(+)-Perillyl Alcohol From (R)-(+)-Limonene Using Engineered Escherichia coli Whole Cell Biocatalyst
Abstract
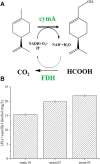
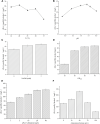
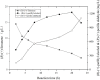
(R)-(+)-perillyl alcohol is a much valued supplemental compound with a wide range of agricultural and pharmacological characteristics. The aim of this study was to improve (R)-(+)-perillyl alcohol production using a whole-cell catalytic formula. In this study, we employed plasmids with varying copy numbers to identify an appropriate strain, strain 03. We demonstrated that low levels of alKL provided maximal biocatalyst stability. Upon determination of the optimal conditions, the (R)-(+)-perillyl alcohol yield reached 130 mg/L. For cofactor regeneration, we constructed strain 10, expressing FDH from Candida boidinii, and achieved (R)-(+)-perillyl alcohol production of 230 mg/L. As a result, 1.23 g/L (R)-(+)-perillyl alcohol was transformed in a 5 L fermenter. Our proposed method facilitates an alternative approach to the economical biosynthesis of (R)-(+)-perillyl alcohol.
Keywords: (R)-(+)-perillyl alcohol; NADH regeneration; alkL; escherichia coli; whole cell catalysis.
Copyright © 2022 Sun, Zhang and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Agulló L., Romero-Silva M. J., Domenech M., Seeger M. (2017). p-Cymene Promotes its Catabolism through the P-Cymene and the P-Cumate Pathways, Activates a Stress Response and Reduces the Biofilm Formation in Burkholderia Xenovorans LB400. PLoS One 12 (1), e0169544. 10.1371/journal.pone.0169544 - DOI - PMC - PubMed
-
- Badee A. Z. M., Helmy S. A., Morsy N. F. S. (2011). Utilisation of orange Peel in the Production of α-terpineol by Penicillium digitatum (NRRL 1202). Food Chem. 126 (3), 849–854. 10.1016/j.foodchem.2010.11.046 - DOI
-
- Berger R. G., De Bont J. A. M., Eggink G., Da Fonseca M. M., Gehrke M., Gros J.-B., et al. (1999). “Biotransformations in the Flavour Industry,” in Current Topics in Flavours and Fragrances: Towards a New Millennium of Discovery. Editor Swift KAD. (Dordrecht: Springer Netherlands; ), 139–170. 10.1007/978-94-011-4022-5_8 - DOI
-
- Bicas J. L., de Quadros C. P., Néri-Numa I. A., Pastore G. M. (2010a). Integrated Process for Co-production of Alkaline Lipase and (R)-(+)-α-terpineol by Fusarium Oxysporum . Food Chem. 120 (2), 452–456. 10.1016/j.foodchem.2009.10.037 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

