Cabotegravir, the Long-Acting Integrase Strand Transfer Inhibitor, Potently Inhibits Human T-Cell Lymphotropic Virus Type 1 Transmission in vitro
- PMID: 35547224
- PMCID: PMC9082600
- DOI: 10.3389/fmed.2022.889621
Cabotegravir, the Long-Acting Integrase Strand Transfer Inhibitor, Potently Inhibits Human T-Cell Lymphotropic Virus Type 1 Transmission in vitro
Abstract
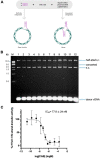
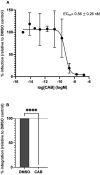
Human T-cell lymphotropic virus type 1 (HTLV-1) is a deltaretrovirus most prevalent in southwestern Japan, sub-Saharan Africa, Australia, South America, and the Caribbean. Latest figures approximate 10 million people worldwide to be infected with HTLV-1. This is likely a significant underestimation due to lack of screening in endemic areas and absence of seroconversion symptoms. The two primary diseases associated with HTLV-1 infection are adult T cell leukaemia-lymphoma, a malignant and, sometimes, aggressive cancer; and HTLV-1 associated myelopathy/tropical spastic paraparesis, a debilitating neurological degenerative disease. Unfortunately, despite the poor prognosis, there is currently no effective treatment for HTLV-1 infection. We previously showed that integrase strand transfer inhibitors (INSTIs) clinically used for human immunodeficiency virus type 1 (HIV-1) prophylaxis and treatment are also effective against HTLV-1 transmission in vitro. In 2021 a new INSTI, cabotegravir, was approved by the FDA for HIV-1 treatment. We thus set out to evaluate its efficacy against HTLV-1 infection in vitro. Strand transfer assays performed using recombinant HTLV-1 integrase treated with increasing concentrations of cabotegravir, effectively inhibited strand transfer activity, displaying an IC50 of 77.8 ± 22.4 nM. Furthermore, cabotegravir blocked HTLV-1 transmission in tissue culture; we determined an EC50 of 0.56 ± 0.26 nM, similar to bictegravir. Alu-PCR confirmed the block in integration. Thus, there are four INSTIs and one reverse transcriptase inhibitor approved by the FDA for HIV-1 treatment, that potently block HTLV-1 infection in vitro. This should strongly encourage the establishment of a new standard of HTLV-1 treatment - particularly for pre-exposure prophylaxis and prevention of mother-to-child transmission.
Keywords: HTLV-1; INSTI; MTCT; cabotegravir; integrase; pre-exposure prophylaxis; tenofovir disoproxil fumarate.
Copyright © 2022 Schneiderman, Barski and Maertens.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cell Culture Evaluation Hints Widely Available HIV Drugs Are Primed for Success if Repurposed for HTLV-1 Prevention.Pharmaceuticals (Basel). 2024 Jun 5;17(6):730. doi: 10.3390/ph17060730. Pharmaceuticals (Basel). 2024. PMID: 38931397 Free PMC article.
-
Inhibition of HTLV-1 Infection by HIV-1 First- and Second-Generation Integrase Strand Transfer Inhibitors.Front Microbiol. 2019 Aug 13;10:1877. doi: 10.3389/fmicb.2019.01877. eCollection 2019. Front Microbiol. 2019. PMID: 31474960 Free PMC article.
-
HTLV-1 Transmission and HIV Pre-exposure Prophylaxis: A Scoping Review.Front Med (Lausanne). 2022 Apr 29;9:881547. doi: 10.3389/fmed.2022.881547. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35572998 Free PMC article.
-
A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection.Retrovirology. 2022 Oct 22;19(1):22. doi: 10.1186/s12977-022-00608-1. Retrovirology. 2022. PMID: 36273165 Free PMC article. Review.
-
Antiviral Activity of Bictegravir and Cabotegravir against Integrase Inhibitor-Resistant SIVmac239 and HIV-1.Antimicrob Agents Chemother. 2017 Nov 22;61(12):e01695-17. doi: 10.1128/AAC.01695-17. Print 2017 Dec. Antimicrob Agents Chemother. 2017. PMID: 28923862 Free PMC article.
Cited by
-
Current Interventions to Prevent HTLV-1 Mother-to-Child Transmission and Their Effectiveness: A Systematic Review and Meta-Analysis.Microorganisms. 2022 Nov 10;10(11):2227. doi: 10.3390/microorganisms10112227. Microorganisms. 2022. PMID: 36363819 Free PMC article.
-
New Perspectives about Drug Candidates Targeting HTLV-1 and Related Diseases.Pharmaceuticals (Basel). 2023 Nov 2;16(11):1546. doi: 10.3390/ph16111546. Pharmaceuticals (Basel). 2023. PMID: 38004412 Free PMC article. Review.
-
Hijacking Host Immunity by the Human T-Cell Leukemia Virus Type-1: Implications for Therapeutic and Preventive Vaccines.Viruses. 2022 Sep 20;14(10):2084. doi: 10.3390/v14102084. Viruses. 2022. PMID: 36298639 Free PMC article. Review.
-
Cell Culture Evaluation Hints Widely Available HIV Drugs Are Primed for Success if Repurposed for HTLV-1 Prevention.Pharmaceuticals (Basel). 2024 Jun 5;17(6):730. doi: 10.3390/ph17060730. Pharmaceuticals (Basel). 2024. PMID: 38931397 Free PMC article.
-
Pre-Exposure Prophylaxis for viral infections other than HIV.Infez Med. 2022 Sep 1;30(3):362-371. doi: 10.53854/liim-3003-5. eCollection 2022. Infez Med. 2022. PMID: 36148176 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

