CGRP Administration Into the Cerebellum Evokes Light Aversion, Tactile Hypersensitivity, and Nociceptive Squint in Mice
- PMID: 35547239
- PMCID: PMC9082264
- DOI: 10.3389/fpain.2022.861598
CGRP Administration Into the Cerebellum Evokes Light Aversion, Tactile Hypersensitivity, and Nociceptive Squint in Mice
Abstract
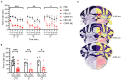
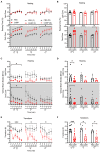
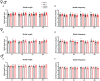
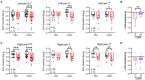
The neuropeptide calcitonin gene-related peptide (CGRP) is a major player in migraine pathophysiology. Previous preclinical studies demonstrated that intracerebroventricular administration of CGRP caused migraine-like behaviors in mice, but the sites of action in the brain remain unidentified. The cerebellum has the most CGRP binding sites in the central nervous system and is increasingly recognized as both a sensory and motor integration center. The objective of this study was to test whether the cerebellum, particularly the medial cerebellar nuclei (MN), might be a site of CGRP action. In this study, CGRP was directly injected into the right MN of C57BL/6J mice via a cannula. A battery of tests was done to assess preclinical behaviors that are surrogates of migraine-like symptoms. CGRP caused light aversion measured as decreased time in the light zone even with dim light. The mice also spent more time resting in the dark zone, but not the light, along with decreased rearing and transitions between zones. These behaviors were similar for both sexes. Moreover, significant responses to CGRP were seen in the open field assay, von Frey test, and automated squint assay, indicating anxiety, tactile hypersensitivity, and spontaneous pain, respectively. Interestingly, CGRP injection caused significant anxiety and spontaneous pain responses only in female mice, and a more robust tactile hypersensitivity in female mice. No detectable effect of CGRP on gait was observed in either sex. These results suggest that CGRP injection in the MN causes light aversion accompanied by increased anxiety, tactile hypersensitivity, and spontaneous pain. A caveat is that we cannot exclude contributions from other cerebellar regions in addition to the MN due to diffusion of the injected peptide. These results reveal the cerebellum as a new site of CGRP actions that may contribute to migraine-like hypersensitivity.
Keywords: CGRP; anxiety; cerebellum; light aversion; migraine; pain.
Copyright © 2022 Wang, Duong, Rea, Waite, Huebner, Flinn, Russo and Sowers.
Conflict of interest statement
AR is a consultant for Lundbeck, Amgen, Novartis, Eli Lilly, AbbVie, and Schedule 1 Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

